Graham’s Law of Diffusion Definition
“The rates of diffusion of two gases are inversely proportional to the square roots of their densities or molecular weights at the same temperature and pressure”.
Mathematical formula:
Graham’s Law of Diffusion Equation,
Where r1 and r2 are the rates of diffusion of two gases, d1 and d2 are their densities and M1 and M2 are the molecular weights of the two gases.
Experimental Verification Of Graham’s Law Of Diffusion Of Gases
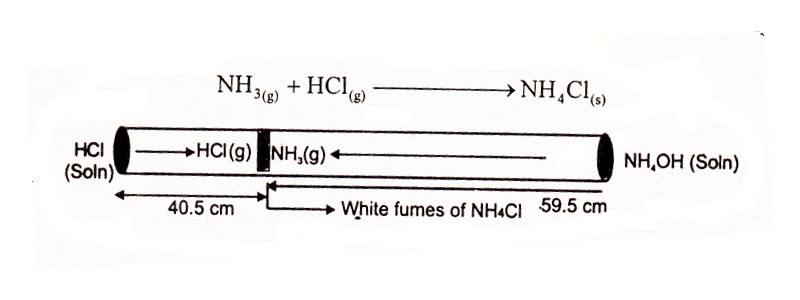
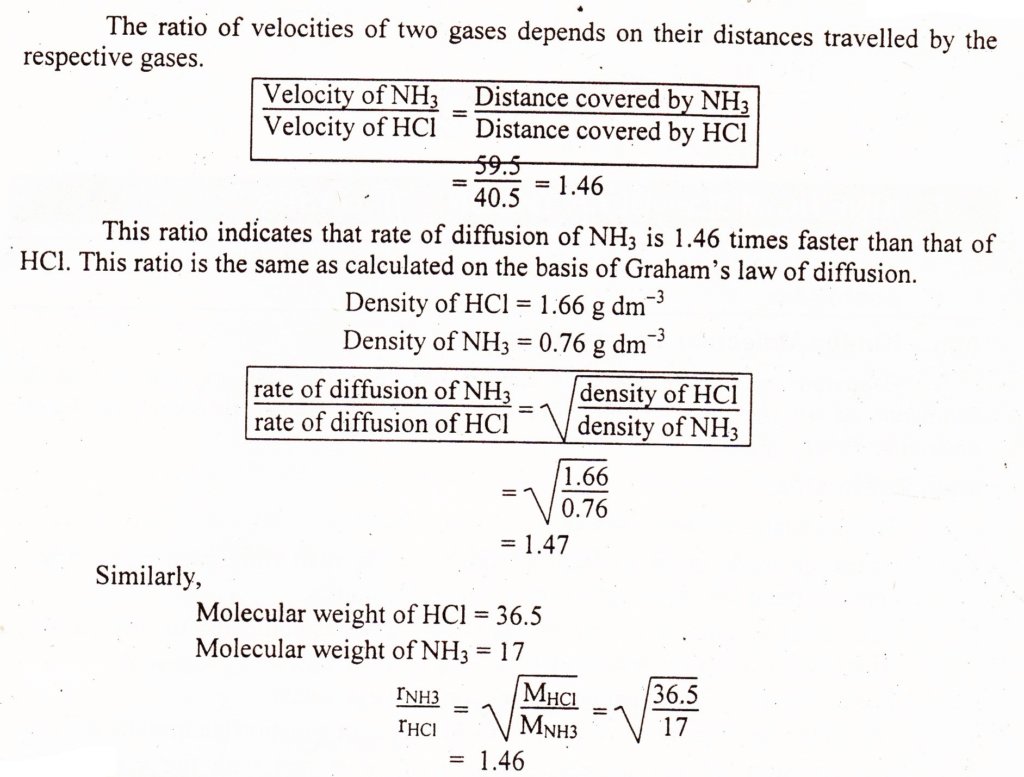
Take 100 cm long glass tube. Plug a cotton swab soaked in HCl at one end and another soaked in NH3 at the other end of the tube simultaneously. The two gases will escape from their solutions and meet at a distance of roughly 59.5 cm from NH3 plug and 40.5 cm from HCl plug, where they form white fumes of NH4Cl.