Petri dish in chemistry is extensively used for…
The solvent in Chemistry is one of the components of a solution, generally the one present in greater quantity or the one that, in the pure state,
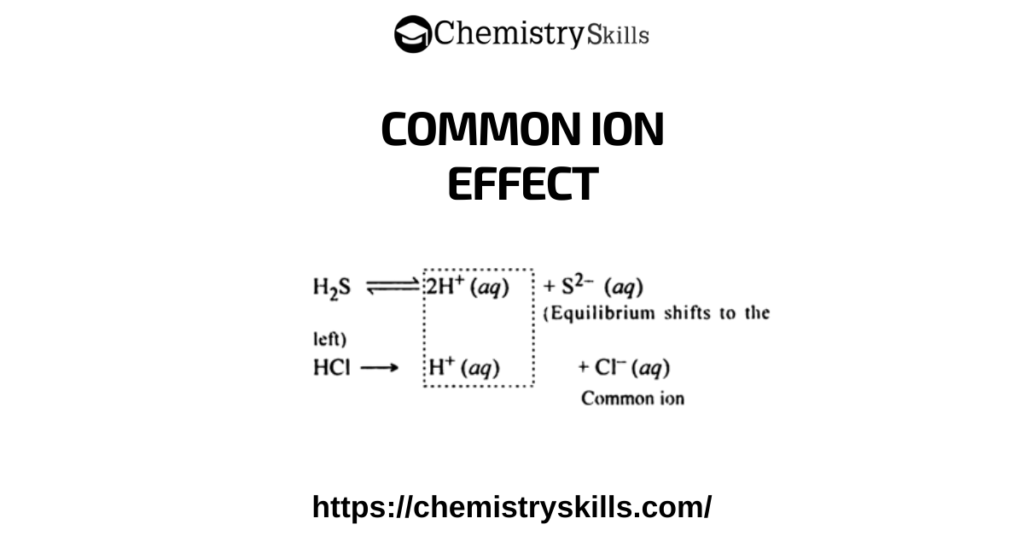
What is Buffer: The solutions, which resist the change in their pH, when a small amount of acid or a base is added to them, are called buffer solutions.
Environmental science is an interdisciplinary science, which involves to multiple sciences. The Environmental Chemistry describes..
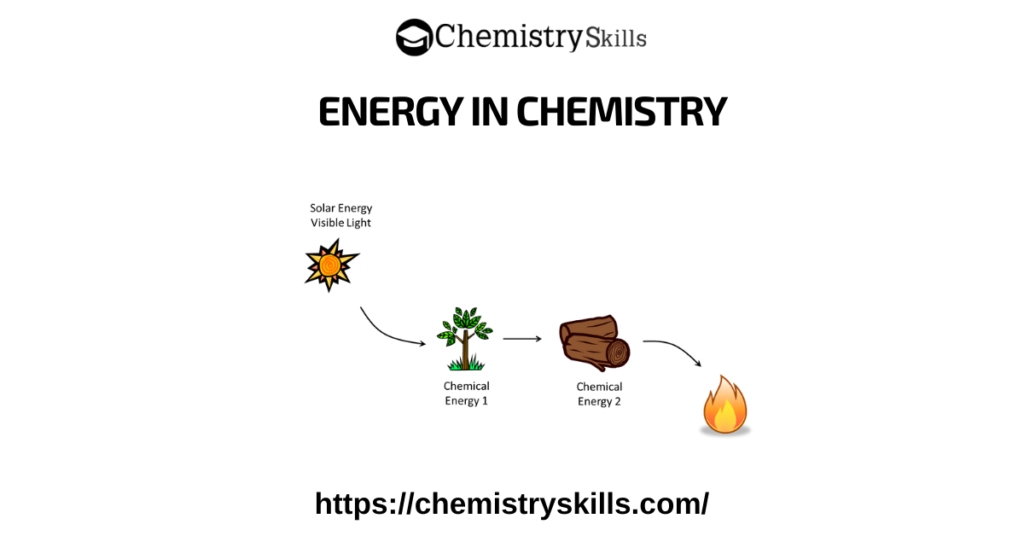
Energy in Chemistry: Energy is a physical quantity that gives a system the ability to do work. It is present in systems in different forms and can