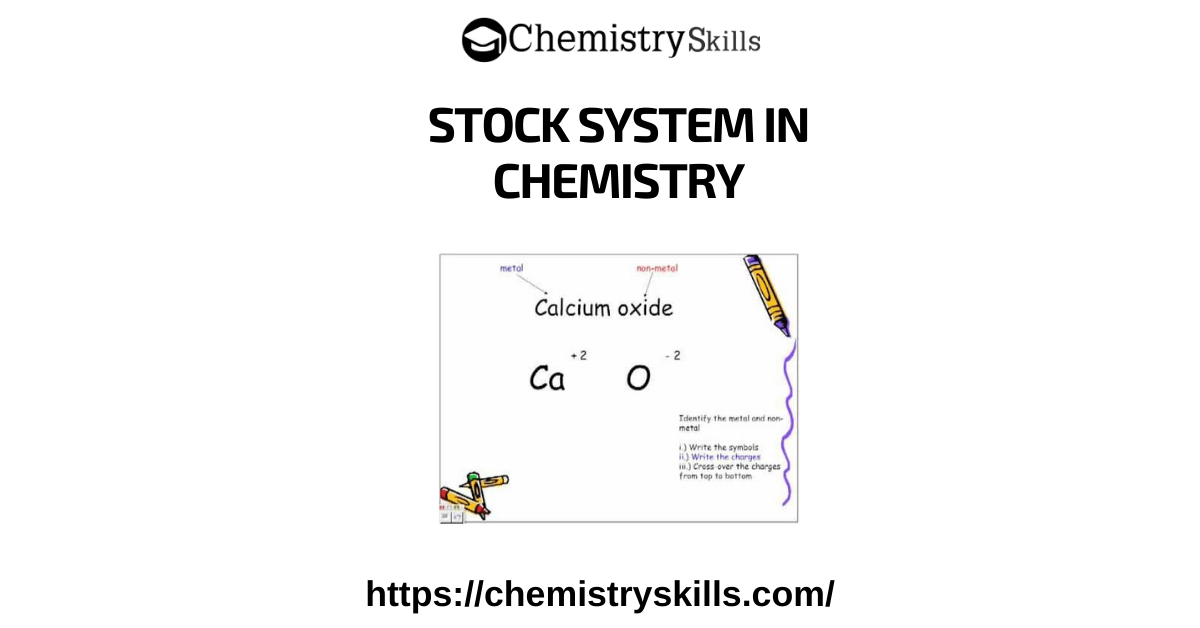
EMPIRICAL FORMULA DEFINITION
A formula which tells the relative number of atoms present in a compound is known as empirical formula.
Examples: NaCl, HO and CH2O are the empirical formulas of sodium chloride, hydrogen peroxide and glucose respectively.
Determination of Empirical Formula for Organic Compounds:
To determine empirical formula percentage of each element present in a compound is required. It is determined by combustion analysis.