Definition of Octane Number
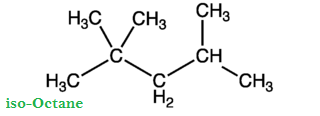
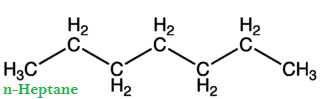
The percentage by volume of iso-octane and n-heptane with the same knocking ability as the fuel under test is called Octane Number of fuel.
ALTERNATIVELY “As gasoline is usually a mixture of n-heptane and iso-octane molecules, thus the octane no. of fuel (gasoline or petrol) can also be defined as
“The number of iso-octane molecules, present in 100 molecules of gasoline, is known as octane number of the fuel or gasoline”.
Greater is the octane number of fuel; excellent will be its quality and vice versa. For example, if we have fuel (gasoline), it has 90% iso-octanes and 10% n-heptane. Then the octane number of this fuel is 90. With an increase of octane number, the engine is less likely to produce knocking. Straight chain hydrocarbons have low octane number and are poor fuels.
Experiments have shown that iso-octane (or 2, 2, 4-trimethyl pentane) burns very smoothly in an engine and has been arbitrarily given an octane number of 100. For improving the octane number of a fuel (i-e converting n-heptane to iso-octane molecules), a process is used, which is called as Refining.
KNOCKING:
Gasoline is a mixture of n-heptane and iso-octane. The straight-chain hydrocarbons are easily ignited (i.e., straight-chain hydrocarbons undergo rapid combustion) compared to branched-chain hydrocarbon. Thus in low octane fuel (gasoline containing low octane number, i.e., higher percentageage of n-heptane), pre-ignition of n-heptane molecule starts sharp metallic sound is produced in the internal combustion engine of the vehicle.
So, this sharp metallic sound produced in the vehicle’s internal combustion engine due to low octane fuel is known as “Knocking.”
To overcome the problem of knocking, two methods are applied:
1) Anti Knocking Agents:
The knocking in the combustion engine of vehicles can be reduced by using an anti-knocking material. An anti-knocking substance is a compound or element, which slows down the combustion reaction of n-heptane of the low octane fuel. Thus, pre-ignition of n-heptane molecules does not occur anymore in the presence of an anti-knocking agent. Therefore, it is clear that an anti-knocking agent acts as a “negative catalyst,” as it slows down the combustion reaction of n-heptane. The most common anti-knocking agent is tetraethyl lead ((C2H5)4Pb). However, the use of tetraethyl lead (TEL) as an anti-knocking agent has a major disadvantage. The combustion product of TEL, i.e., lead oxide, is reduced to metallic lead (Pb), which is discharged into the air through the exhaust pipe and causes air pollution. Therefore, the use of TEL as an anti-knocking is nowadays banned.
2) Reforming of Petrol:
The conversion of straight-chain hydrocarbon into branched-chain hydrocarbons by heat in the absence of oxygen and the presence of a catalyst is called Refining.
The process known as the Reforming of Petrol, enhances the octane number of low octane petrol (Gasoline). In this process, the n-heptane molecules are converted into iso-octane molecules by high heating in the presence of a catalyst, and thus the octane number of gasoline is enhanced and knocking problem is reduced in minimum, i.e.,
After Reforming,