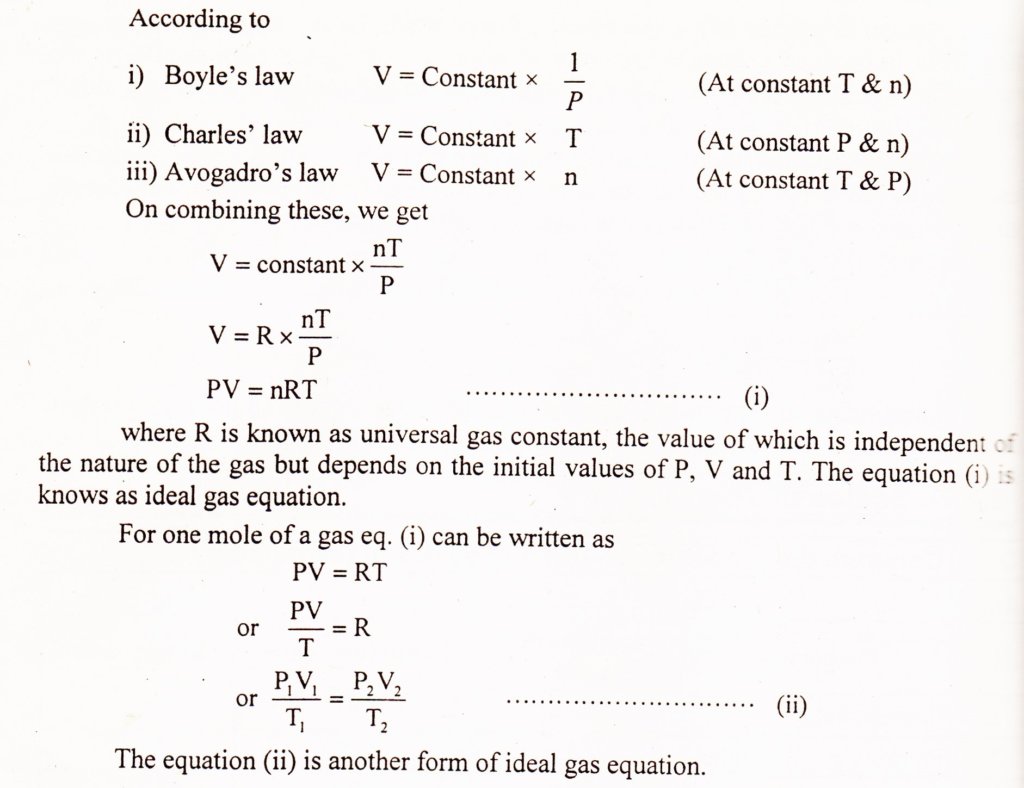
Definition:
The relationship, which shows the effect of simultaneous changes in pressure and temperature on the volume of given mass of a gas is called ideal gas law or combined gas law.
Mathematical Form: (General gas equation)
Mathematically, it is expressed as
PV = nRT
This equation is derived by combining Boyle’s law, Charles’ law and Avogadro’s law as given below: