Definition:
The attractive forces between the positive end of one molecule with the negative end of other molecule are called dipole-dipole forces.
Explanation:
In case of HCl, There is much electronegativity difference between hydrogen and chlorine. Due to this chlorine being more electronegative develops a partial negative charge whereas hydrogen develops partial positive charge. Hence the molecules are close to each other, the positive end of one molecule attracts the negative end of the other molecule. They tend to line up. There is a net attraction between the polar molecules. These forces are called as dipole-dipole forces and they are approximately one per cent as effective as covalent bond.
The strength of these forces depends upon the electronegativity difference between bonded atoms and the distance between the molecules. The distances between molecules in the gaseous phase are greater so these forces are very weak in this phase. The examples of the molecules which show dipole-dipole attractions are given below:
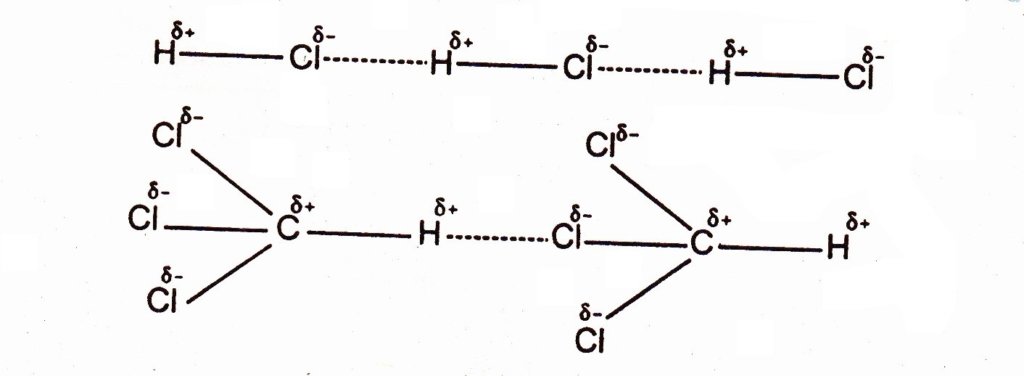
Stronger these dipole-dipole forces, greater would be the value of thermodynamic parameters like melting point, boiling point, heat of vaporization and heat of sublimation.



