In Bohr’s atom, the electrons are moving with specific velocities in orbits of specified radii, and according to the uncertainty principle, both these quantities cannot be measured experimentally. A theory involving quantities, which cannot be measured, does not solve this difficulty, Schrödinger,
0.0529 nm = 0.529Ao
Heisenberg and Dirac worked out wave theories of the atom. The best-known treatment is that of Schrödinger.
Schrödinger set up a wave equation for the hydrogen atom. According to Schrödinger, although the position of an electron cannot be found exactly, the probability of finding an electron at a particular position at any time can be found.
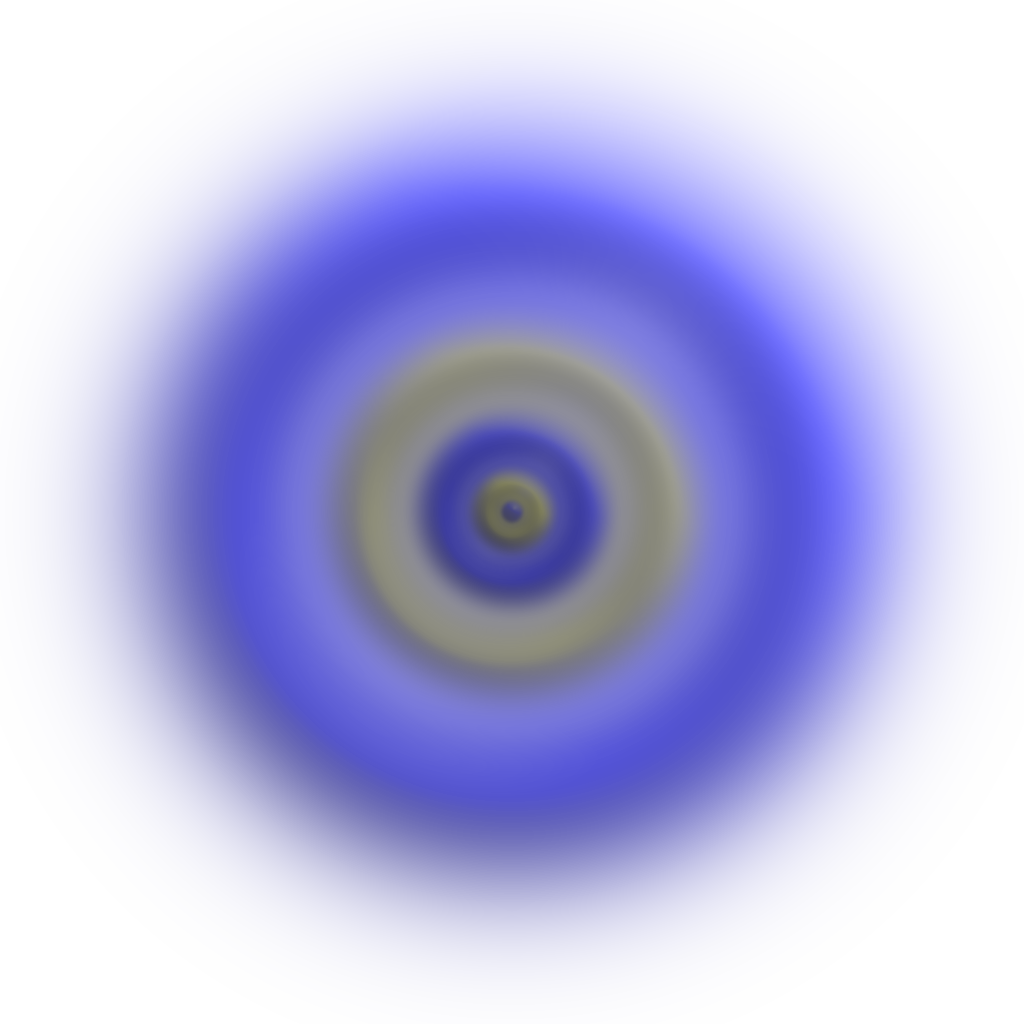
The maximum probability of finding the electron is at a distance of 0.053 nm. It is the same radius as calculated for the Bohr’s first orbit. There is a possibility that the electron is either closer to the nucleus or outside the radius of 0.053 nm, where the probability of finding electron decreases sharply.
“The volume of space in which there is a 95% chance of finding an electron is called an atomic orbital.”
The orbital can be regarded as a spread of charge surrounding the nucleus. This is often called the ‘electron cloud’.