The number of atoms, molecules, formula units or ions in one gram atom, one gram molecule, one gram formula unit or one gram ion, respectively is called Avogadro’s number.
OR
The number of atoms, molecules, formula units or ions in one mole of the substance is called Avogadro’s number.
Representation of Avogadro’s Number:
It is represented by NA.
Value of Avogadro’s Number:
Its value is 6.02 x 1023.
Examples:
- 1 gram of H = 1 gram atom of H = 1 mole of H = 6.02 x 1023 atoms of H
- 2 grams of H2 = 1 gram molecule of H2= 1 mole of H2 = 6.02 x 1023 molecules of H2
- 58.5 grams of NaCl = 1 gram formula mass of NaCl= 1 mole of NaCl = 6.02 x 1023 formula units of NaCl
- 23 grams of Na+1= 1 gram ion of Na+1 = 1 mole of Na+1 = 6.02 x 1023 ions of Na+1
Formulas, which can be used to calculate the number of atoms, molecules, formula units or ions in the given sample of a substance are given below:
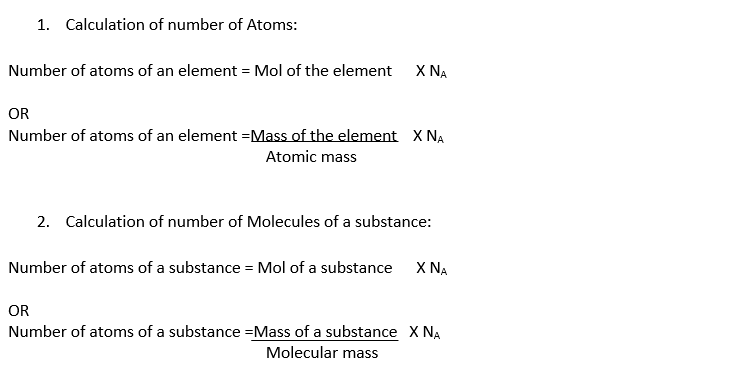
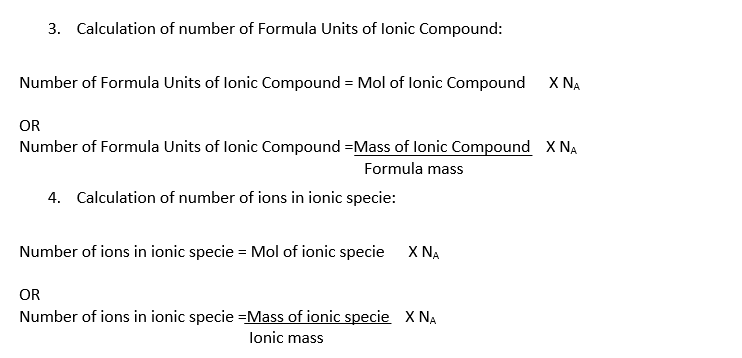
Also Read: Avogadro’s Law



