Dalton’s Law Of Partial Pressure
Statement:
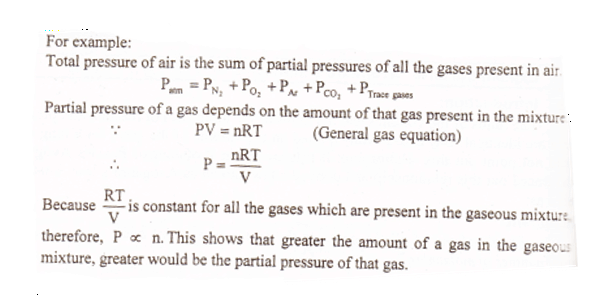
“The total pressure exerted by a mixture of gases is the sum of the partial pressure of all the gases present in the mixture”.
Dalton’s law of partial pressure formula/Equation:
Mathematically,
P= P1 + P2 + P3 – – – – – – –
Where P1, P2 and P3 are the partial pressures of different gases and P is the total pressure of the gases present in the gaseous mixture.
The pressure exerted by each gas in the gaseous mixture is called the partial pressure of the gas. Dalton’s law of partial pressure Mathematical Expression:
Application of Dalton’s Law of Partial Pressure
1. Respiration under normal conditions:
The respiration takes places in living organism due to the difference in partial pressure. Oxygen moves from the air where its partial pressure is high (159 mm Hg) to the lungs where its partial pressure is lower (116 mm Hg). The carbon dioxide, which is the waste product of respiration, moves in the opposite direction and is exhaled into the atmosphere.
2. Respiration at high altitude:
One can respire comfortably when partial pressure of oxygen is 159 mm Hg. At high altitude where the partial pressure of oxygen becomes lower than 159 mm Hg, the pilots may have uncomfortable breathing in non-pressurized cabin.
3. Respiration under sea water:
Sea water exerts pressure and causes to increase the partial pressure of oxygen used by sea divers. In deep sea diver’s tank, inert gas is mixed with oxygen to adjust the partial pressure of oxygen according to requirements.
4. Collection of gas over water:
Gases collected over water contain some water vapour. Partial pressure of such gases is calculated by using the following mathematical expression of Dalton’s law of partial pressure.
P total = P gas + P water vapour
P gas = P total – P water vapour
The partial pressure of water vapour in gases is called aqueous tension or vapour pressure of water.
The vapour pressure of water is different at different temperatures.