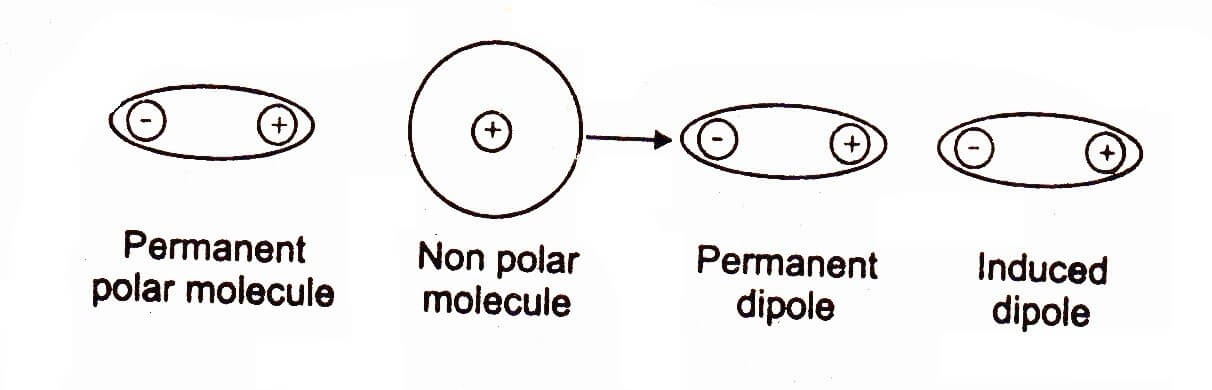
Definition:
The forces of attraction between polar molecule and temporarily induced molecule (neutral) are called dipole-induced dipole or Debye forces.
Explanation:
In certain cases, we have a mixture of substances containing polar and non-polar molecules. The positive end of the polar molecule attracts the mobile electrons of the nearly non-polar molecule. In this way polarity is induced in non-polar molecule, and both molecules become dipoles.
These forces are also called as Debye forces. The following figure makes the idea clear. For example when H-Cl and argon are mixed together, they attract with dipole induced dipole forces.