How to Use the Stock System in Chemistry?
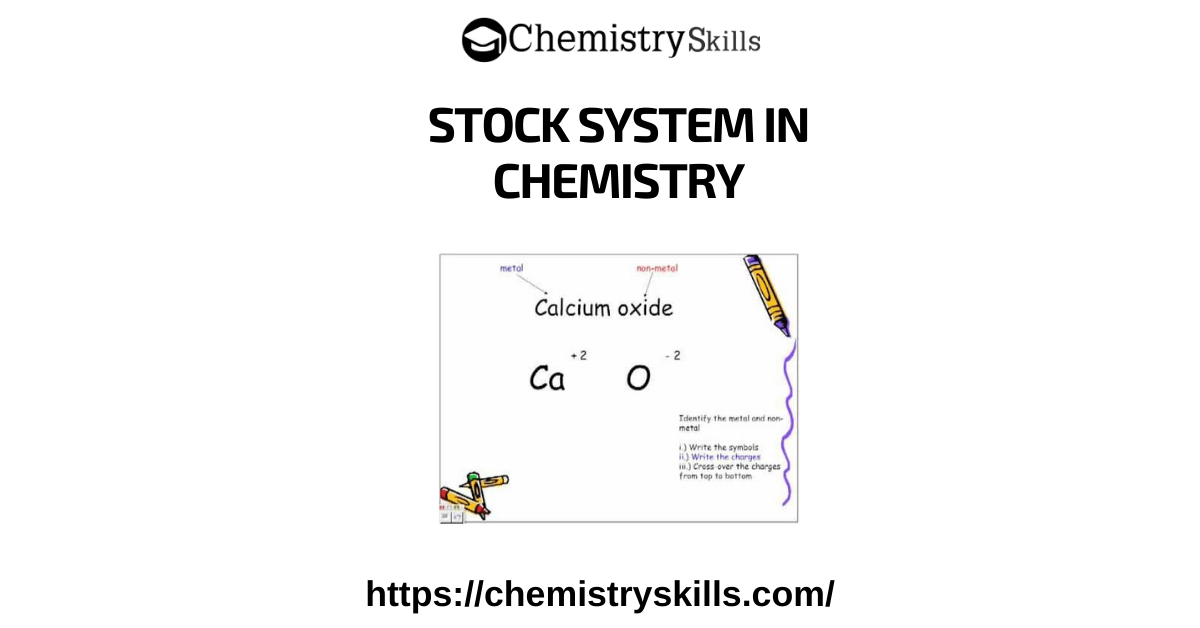
The stock system in chemistry is used for naming ionic compounds.
There are some metals, which actually have more than one possible oxidation state.
The stock system let us know which version of that metal ion we are considering.
For example, copper can be either from a Cu+1 or Cu+2.
Similarly, iron can form Fe+2 or Fe+3.
Some other examples of this both tin and lead can form Sn+2, Sn+4 & Pb+2, Pb+4.
I suggest you memorize the oxidation states of metals, which have more than one possible oxidation state.
Now let us look at examples of Sn,
Tin (Sn) reacts with Chlorine (Cl) to give either SnCl2 or SnCl4.
In SnCl2, Tin (Sn) has an oxidation state of +2, while in SnCl4 oxidation state is +4.
For detail and example, please read the following article,