London Dispersion Forces
Definition:
The momentary forces of attraction which are created between and induced dipole is called Instantaneous dipole-induced dipole forces or London Dispersion Forces.
Or
The forces of attraction between non-polar molecules which are formed polar for an instant are called instantaneous dipole-induced dipole forces or London forces.
Explanation:
The forces of attraction present among the non-polar molecules like helium, neon, argon, chlorine and methane need special attention because under the normal conditions such molecules don’t have dipoles. We know that helium gas can be liquefied under appropriate conditions. In other words forces of attraction operate among the atoms of helium which cause them to cling together in the liquid state.
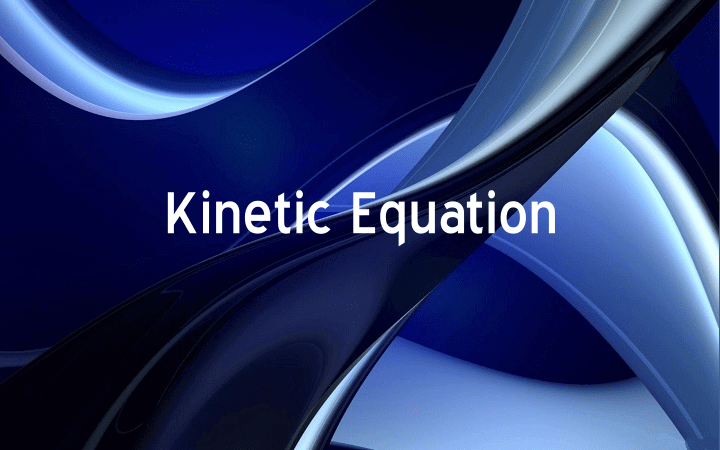
In helium gas, the electrons of one atom influence the moving electrons of the other atom. Electrons repel each other and they tend to stay as far apart as possible. When the electrons of one particle go near to the electrons of other atom, they are pushed away each other. In this way a temporary dipole is created in the atom.
The result is that, at any moment the electron density of the atom is no more symmetrical. It has more negative charge at one side than on the other side. At that particular instant, the atom becomes a dipole. This is called instantaneous dipole. This instantaneous dipole disturbs the electronic cloud of the other nearby atom. So a dipole is induced in the second atom. This is called induced dipole. The momentary force of attraction created between instantaneous dipole and the induced dipole is called dipole-induced dipole interaction or London forces. It is very short-lived attraction because the electrons keep moving. This movement of electrons cause the dipoles to vanish as quickly as they are formed.
These forces are present in all types of polar and non-polar molecules whether polar or non-polar but they are very significant for non-polar molecules like Cl2, H2 and noble gases.
Factors Which Affect London Dispersion Forces
Factors affecting the London forces are:
(i) Atomic or molecular size (ii) Polarizability (iii) Number of atoms in a molecule.
(i) Atomic or molecular size
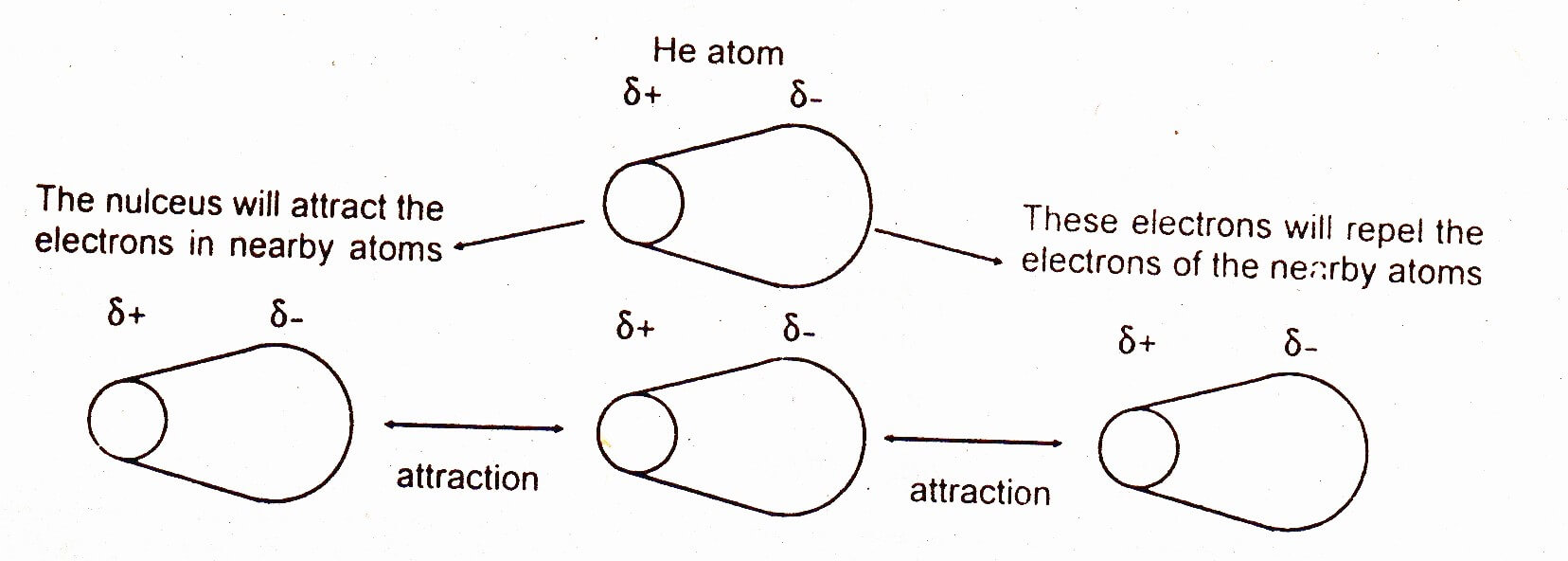
Dipole-dipole interactions are stronger than London forces. The strength of these forces depends upon the size of the electronic cloud of the atom or molecule. When the size of the atom or molecule is larger, then the dispersion becomes easy and these forces become more prominent. The elements of the zero group in the periodic table are atomic gases. They don’t make covalent bonds with other atoms because their outermost shells are complete. Their boiling points increase down the group from helium to radon.
The following graph shows the increase in their boiling points:
(ii) Polarizability
“The quantitative measurement of the polarizability is the extent to which the electronic cloud can be polarized. The increased distortion of electrons creates stronger London forces and hence the boiling points are increased down the group”.
As the atomic number increases down the group the outermost electrons become away from the nuclei. The dispersion of the electronic clouds becomes more and more easy. So the polarizability of these atoms goes on increasing.
Similarly, the boiling points of halogens in group VIIA go on increasing from fluorine to iodine. All the halogens are non-polar diatomic molecules, but there is a big difference in their physical states at room temperature. Fluorine is a gas and boils at188.1°C. The polarizability of iodine molecule is much greater than that of fluorine.
(iii) Number of atoms in a molecule
Another important factor that affects the strength of London forces is the number of atoms in a non-polar molecule. Greater the number of atoms in a molecule, greater is the polarizability of the molecule.