Plank’s Quantum Theory
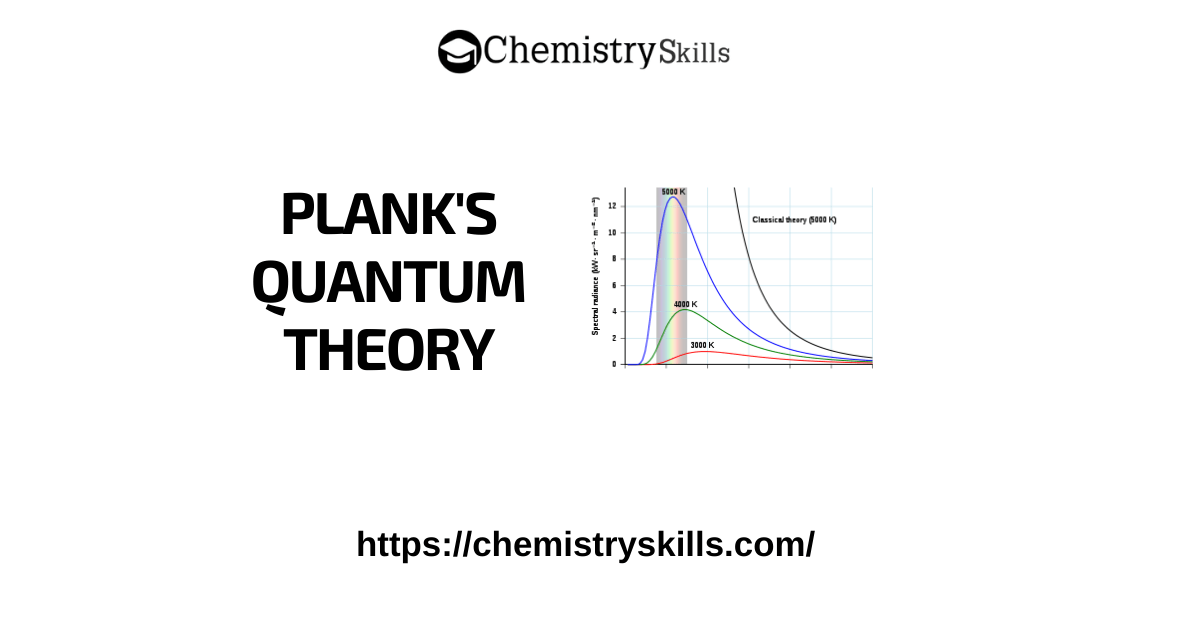
In 1900, Max Planck proposed the quantum theory to explain the emission and absorption of radiations. Heated objects give off these radiations. According to his revolutionary hypothesis, energy travels in a discontinuous manner and is composed of a large number of tiny discrete units called quanta.
Main postulates of Plank’s Quantum Theory:
The main points of the theory are:
1. Energy is not emitted or absorbed continuously. Each wave packet or quantum is associated with a definite amount of energy. In the case of light, the quantum of energy is often called the photon.
2. The amount of energy associated with a quantum of radiation is proportional to the frequency of the radiation.
Ε ∝ ∨
E=h∨
Where ‘h’ is a constant known as Planck’s constant and it is equal to 6.625X10-34 Joule sec.
3. A body can emit or absorb energy only in terms of integral multiples of a quantum.
E = n h ∨
Where
n= 1, 2, 3… (Integral Multiple)
The frequency ‘∨’ is related to the wavelength ‘λ’ of the photon as.
∨ = c/λ.
E = h c/λ