Before understanding the state of dynamic equilibrium, it is important to understand irreversible & reversible reactions.
Irreversible Reactions:
The reactions, which proceed to completion when stoichiometric amounts of the reactants are taken, are called irreversible reactions.
Direction:
Such reactions take place in one direction only.
Example:
Formation of water from hydrogen and oxygen by means of electric spark at normal temperature and pressure is an example of irreversible reaction.
2H2 (g) + O2 (g) → 2H20 (l)
The above reaction proceeds to completion when stoichiometric amounts of hydrogen and oxygen are taken, however reverse of the above reaction is possible but not under the same set of above-mentioned conditions of the reaction.
Reversible Reactions:
The reactions, which do not proceed to completion even when stoichiometric amounts of the reactants are taken, are called reversible reactions. The phenomena of dynamic equilibrium takes place for reversible reactions.
Direction:
Such reactions take place both in the forward and backward directions under the same set of conditions.
Example:
Formation of ammonia from nitrogen and hydrogen at 450°C under high pressure in the presence of iron (Fe) catalyst is an example of reversible reaction.
N2 (g) + 3H2 (g) ⇆2NH3 (g)
The above reaction does not proceed to completion, no matter how long the reaction is allowed to continue. Reverse of the above reaction i.e. decomposition of ammonia to nitrogen and hydrogen is also possible under the same set of abovementioned conditions of the reaction.
State Of Dynamic Equilibrium
The state of a reversible reaction at which the composition of the reaction mixture does not change is called the state of Dynamic equilibrium or chemical equilibrium.
Explanation:
At particular conditions, the composition of the reaction mixture of a particular reversible reaction at equilibrium state is always the same; however, it varies with the change in conditions.
Consider the following general reversible reaction to illustrate the state of chemical equilibrium.
A+B ⇆C + D
Let the initial concentrations of A and B are equal. A reacts with B to produce C and D.
As the reaction proceeds, concentrations of both A and B decrease first rapidly, then slowly. Finally, their concentrations stop to decrease.
Correspondingly, both C and D’s concentration increases first rapidly, then slowly, and finally, their concentrations stop to increase. As the composition of the reaction mixture does not change at this state, it is the equilibrium state.
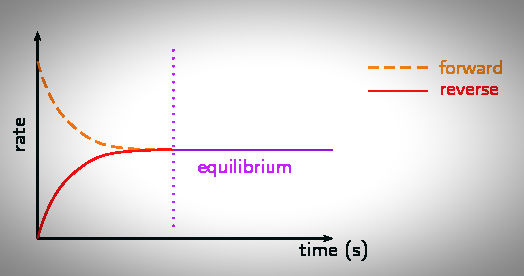
State Of Dynamic Equilibrium for Reversible Reaction
Consider the following reversible reaction between Hydrogen (H2) and Iodine (I2) at 450°C
H2 (g) + I2 (s) ⇆ 2HI (g)
As the reaction proceeds, concentrations of both H2 and 12 decreases first rapidly; then slowly, and finally, their concentration stops to decrease.
Correspondingly, the concentration of HI increases first rapidly, and then slowly, and finally, its concentration stops to increase. As the composition of the reaction mixture does not change at this state, therefore it is The state of Dynamic Equilibrium commonly known as chemical equilibrium. However, it does not mean that reaction has stopped. At this state, reaction proceeds in both forward and backward directions but with equal rates. This is why the rate of chemical equilibrium is known as a dynamic equilibrium state.



