Many elements exist as a mixture of their isotopes. The atomic masses of such elements depend upon the number of their isotopes and their natural abundances. This is why their atomic masses are in fraction.
This can be explained through following example,
EXAMPLE:
A sample of neon is found to consist of 20Ne, 21Ne and 22Ne in the percentages of 90.92%, 0.26% and 8.82%, respectively.
Calculate the fractional atomic mass of neon.
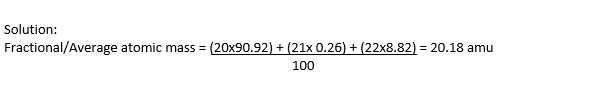
Though no individual neon atom in the sample has mass of 20.18 amu. However, every atom of the sample is considered to have mass 20.18 amu.



