Why ice floats on water?
When the temperature of water is decreased and ice is formed then the molecules become more regular and this regularity extends throughout the whole structure. Empty spaces are left behind as shown in the figure below.
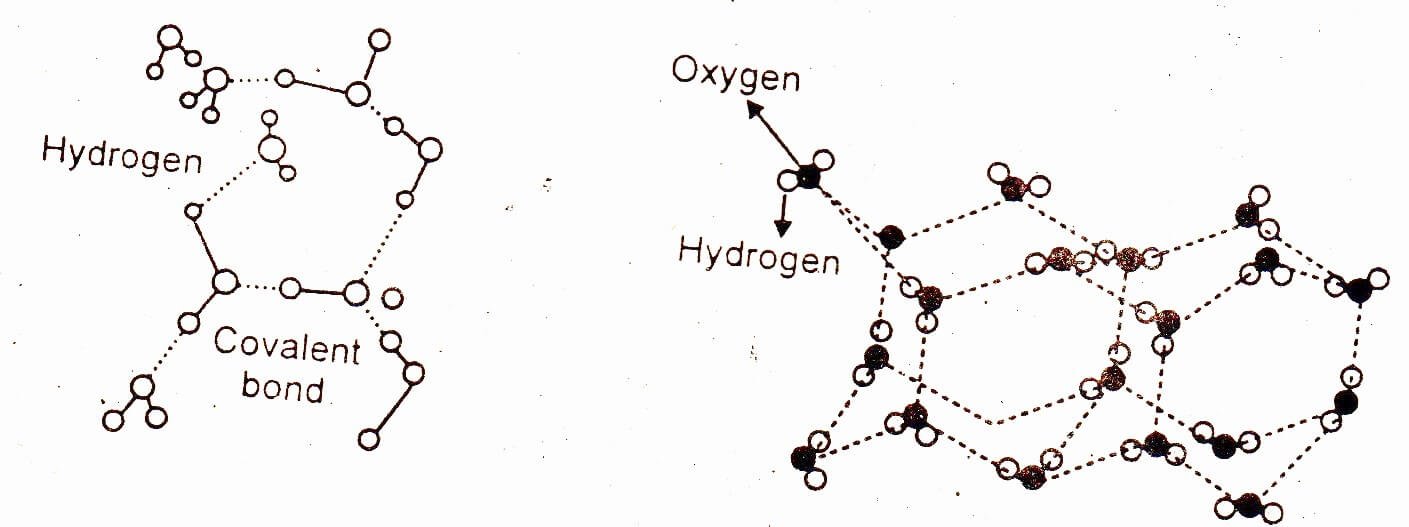
That is why when water freezes it occupy 9% more space and its density decreases. The result is that ice floats on water. The structure of ice is just like that of a diamond because each atom of the carbon in diamond is at the center of tetrahedron just like the oxygen of water molecule in ice.