Define and explain vapour pressure
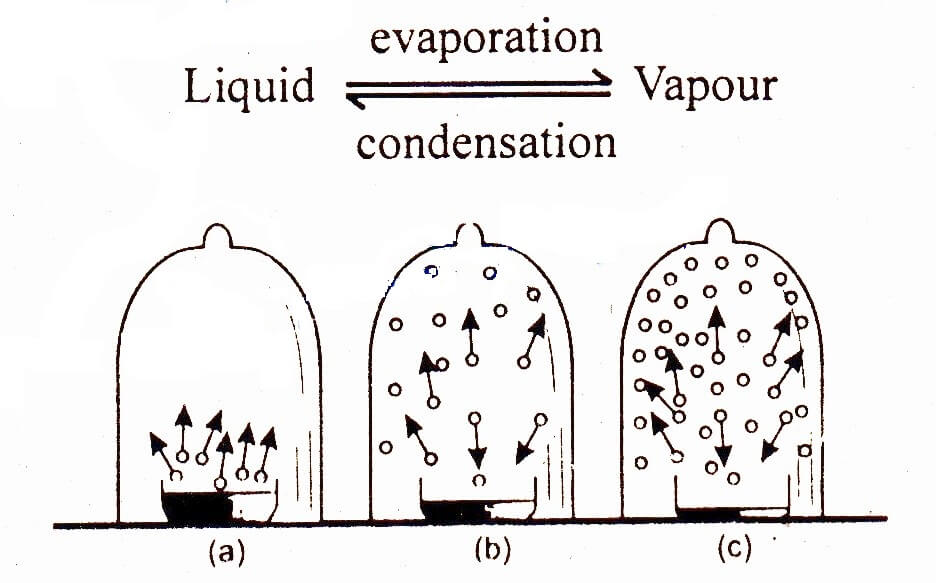
The pressure above the surface of the liquid by the vapours at equilibrium between liquid and its vapours at a particular temperature is called equilibrium vapour pressure or simply vapour pressure.
Explanation:
If we enclosed a liquid in a vessel, molecules start to evaporate above the surface of liquid. Such molecules return back to the liquid as a result of condensation. In the beginning, rate of condensation is smaller than the rate of evaporation. However, after sometime both these rates become equal and equilibrium is established between the liquid and vapour states of the substance.
Factors affecting vapour pressure
Vapour pressure does not depend upon the area or the amount of the liquid nor does it depend upon volume of container.
Following are the factors affecting vapour pressure
-
Nature of the liquid:
Vapour pressure usually depends on liquid’s nature i.e. on sizes of molecules. At same temperature different liquids have different vapour pressure.
-
Intermolecular forces
Weaker the intermolecular forces, greater will be the vapour pressure.
This is because intermolecular attractive forces in diethyl ether are weaker than in ethyl alcohol, which in turn are weaker than water.
-
Temperature
Higher the temperature, greater would be the vapour pressure of the liquid. This is because increase in temperature increases the fraction of the molecules possessing enough kinetic energy to overcome the intermolecular attractive forces.
Measurement of Vapour Pressure
Manometric Method:
Manometric method is used for accurate determination of vapour pressure of a liquid at a particular temperature.
The liquid whose vapour pressure is to be determined is taken in the flask connected with the vertical end of T-shaped tube, one end of which is connected with a manometer and other end is connected with vacuum pump. The liquid is frozen. The air in the flask is removed with the help of the vacuum pump. The frozen liquid is melted so that it may release the air entrapped by it. The liquid is frozen again. The above mentioned released air is removed with the help of vacuum pump. The process is repeated till almost all the air entrapped by the liquid is removed. The flask containing air free liquid is placed in a thermostat adjusted at the desired temperature. The liquid will begin to convert into vapour, which exert pressure on the mercury of manometer. This pressure can be measured with the help of manometer at the temperature adjusted by the thermostat. The difference Ah in mercury column in both the limbs is then noted. The vapour pressure of the liquid is calculated by applying the following equation:

If level of the right hand side mercury column is higher than the left hand side column, whereas in the reverse case, its value would be negative.
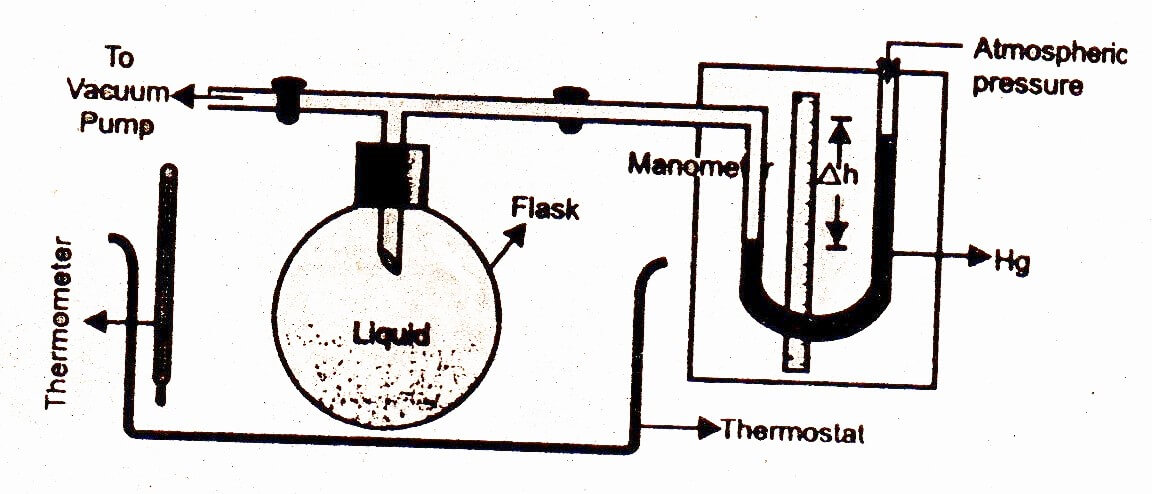
P =Pa+h
Where P = Vapour pressure of the liquid at 1 atm pressure
Pa= Atmospheric pressure
h = Difference in the heights of the mercury levels in the two limbs of the manometer






