Following statement of Charles’ law helps to derive absolute zero of temperature.
“At constant pressure the volume of a given mass of an ideal gas increases or decreases by 1/273 of its original volume at 0°C for every 1°C rise or fall in temperature respectively.”
ABSOLUTE ZERO TEMPERATURE
Definition:
The temperature at which volume of an ideal gas becomes equal to zero is called absolute zero temperature. (Zero of the Kelvin scale.)
O K = -273.16°C
For routine calculations the value of the absolute zero is taken as -273.16°C. None of the real gas can be cooled to such a low temperature i.e. 0 K.
All real gases far above this temperature either liquefy or solidify. The volume of the gases cannot be made zero. This is because it is against the law of conservation of mass.
At absolute zero temperature, volumes of all gases become equal to zero if they do not condense. Below the absolute zero temperature the volume of all the gases will become negative which is impossible. It means absolute zero temperature is the lowest possible temperature.
Graphical explanation of absolute zero temperature:
When a graph is plotted between the temperature on x-axis and the volume of one mole of a gas on y-axis, then a straight line is obtained. On extrapolation this straight line cuts the x-axis i.e. temperature axis at -273.16°C, which shows that volume of given mass of a gas becomes zero at this temperature, however, all gases convert into liquids above this temperature.
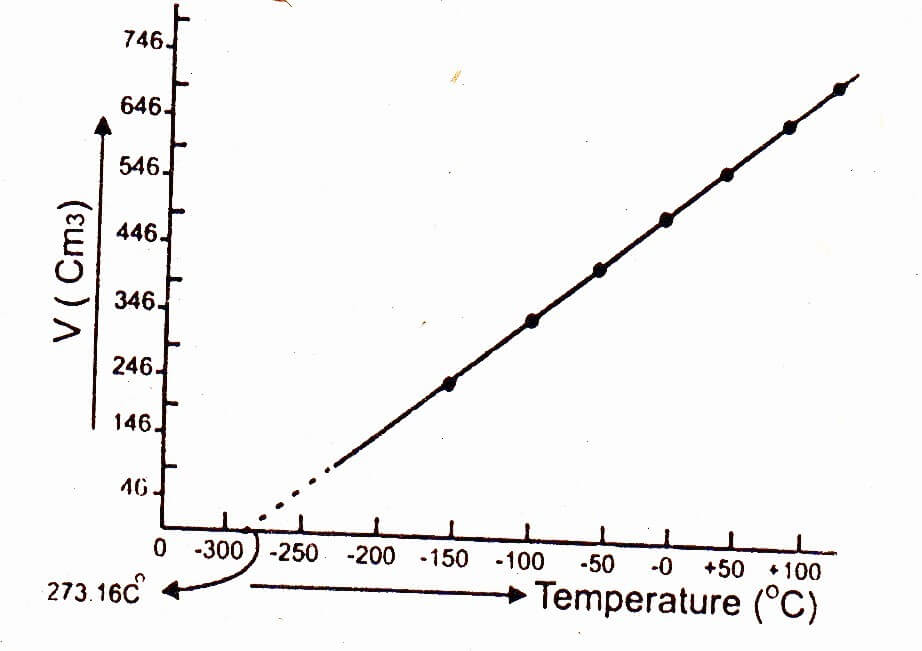
Greater the mass of the gas taken, greater would be the slope of the straight line. This is because greater the number of moles of the gas, greater would be the volume of the gas. However, all the straight lines on extrapolation meet at a single point 0 K (i.e. -273.16°C).
Charles’ law is obeyed when the temperature is taken on the Kelvin’ scale. For example, at 283 K (10°C) the volume of the gas is 566 cm3, whereas the volume of the gas is 746 cm3 at 373 K (100°C).
According to Charles’ law:
V1/T1=V2/T2=K
566/283=746/373=2=k
Substitution of centigrade temperature in the above expression does not produce constant value.



