Millikan, in 1909, determined the charge on electron by the apparatus consisting of a metallic chamber having two parts. The chamber is filled with air whose pressure can be adjusted by a vacuum pump. There are two electrodes A and A’. These electrodes are used to generate an electrical field in the space between the electrodes. The upper electrode has a hole in it.
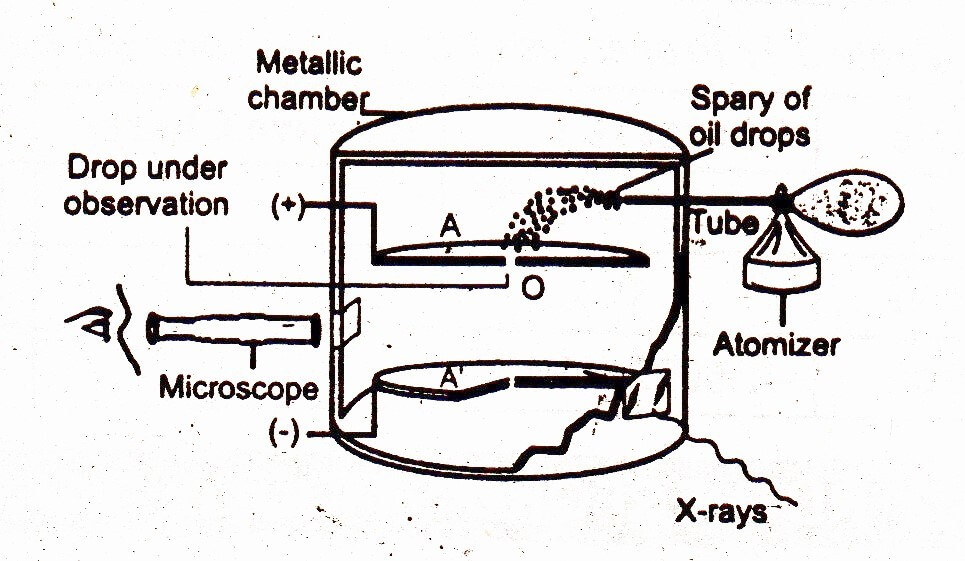
A fine spray of oil droplets is created by an atomizer. A few droplets enter the hole. Then the hole is closed. An arc lamp is used to illuminate the space between the electrodes. The droplet falls under the force of gravity between two plates A and A’. The velocity of the droplet is determined.
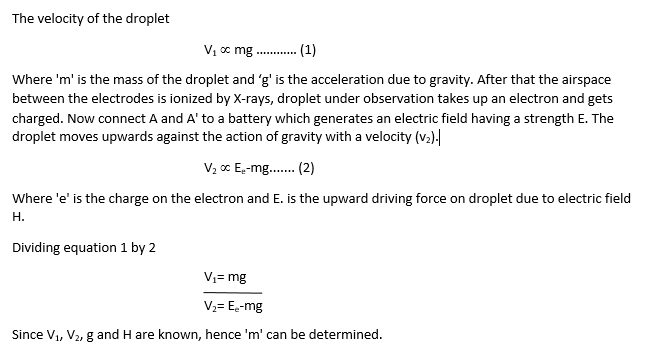
By changing the strength of electrical field Millikan found that the charge on each droplet was different. The smallest charge which he found was 1.59×10-19 coulombs, which is very close to the recent value of 1.6022×10-19 coulombs. The charge taken by the electron is the smallest charge of electricity that has been measured so far.



