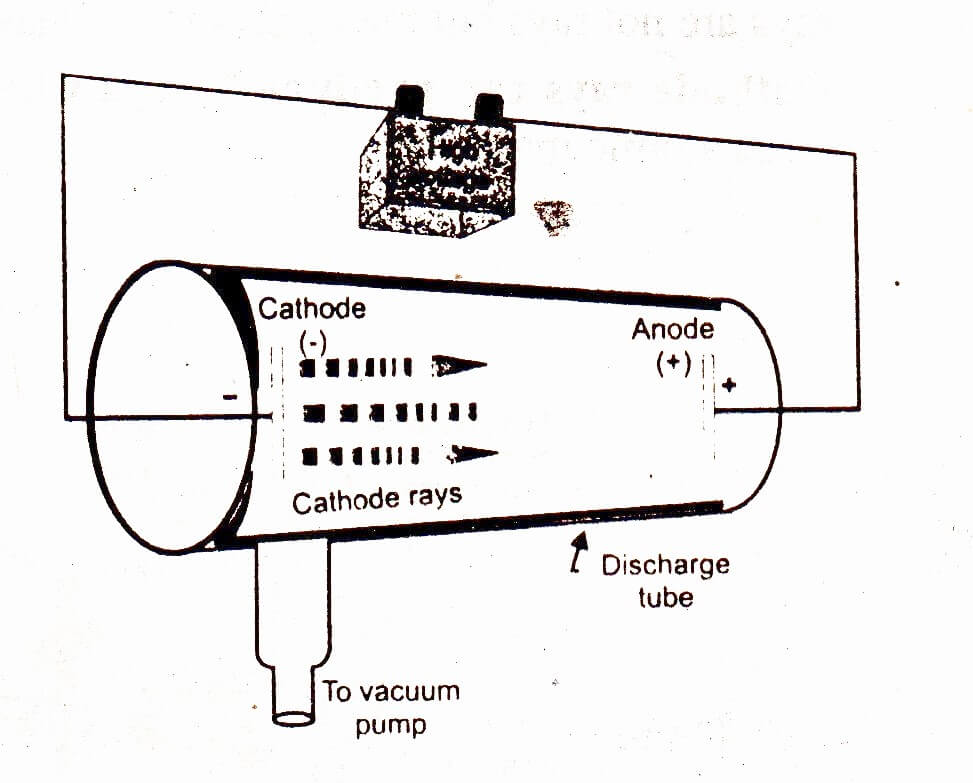
Discharge tube
The discharge tube is a glass tube having two electrodes sealed into it connected to a vacuum pump to reduce the pressure of the gas taken into it. A slit is placed in the tube to get a sharp beam of radiations.
Experiment:
William Crooks a British scientist studied the passage of electricity through gases taken at different pressures in the gas discharge tube. He observed that air taken in the gas discharge tube at ordinary pressure did not allow the electricity to flow, even when a source of high potential of about 5000 Volt was used. However, when pressure of air was reduced by removing most of the air from the discharge tube then it allowed the current to flow and emitted light (as in neon sign). When pressure was reduced further to about 0.01 torr, then emission of light by air ceased. But the current still flows between the electrodes and produced fluorescent on striking the glass walls opposite to the cathode. This was the result of rays emitted by cathode. Rays emitted by cathode when electricity is passed through a gas taken in the discharge tube at very low pressure are called cathode rays.
Discovery of electron
Emission of cathode rays does not depend on the nature of the electrodes or the gas used in the discharge tube. This indicates that cathode rays (i.e., electrons) are the constituents of all types of matter.
Conclusion:
J.J. Thomson calculated the mass of cathode rays. He suggested that these rays are matter and not electromagnetic radiations. He proposed the name corpuscles for these particles. But as these particles were similar to the particles present in on the electricity, therefore, later on they were named electrons. J.J. Thomson won the 1906 Nobel Prize.