To study the properties of cathode rays systematic investigations were made by W. Crooks, J.J. Thomson and J. Perrin.
They established the following properties of cathode rays:
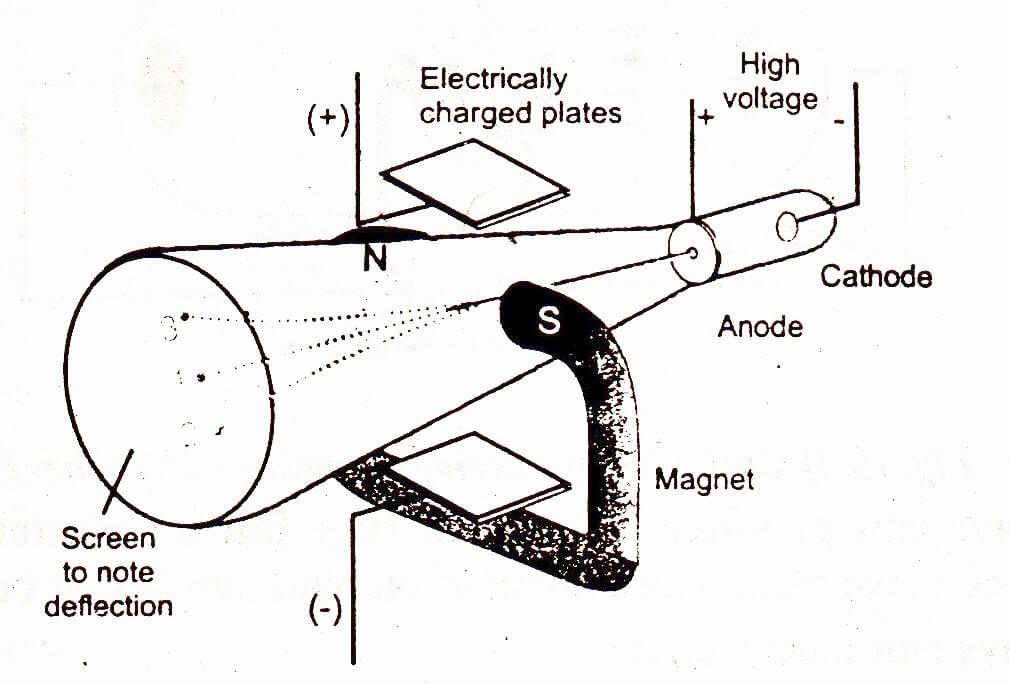
- Cathode rays are negatively charged.
In 1895, J.Perrin showed that cathode rays are deflected in a magnetic field perpendicular to the line joining the two poles. In 1897, J.J. Thomson found electric charge through applying electric field.
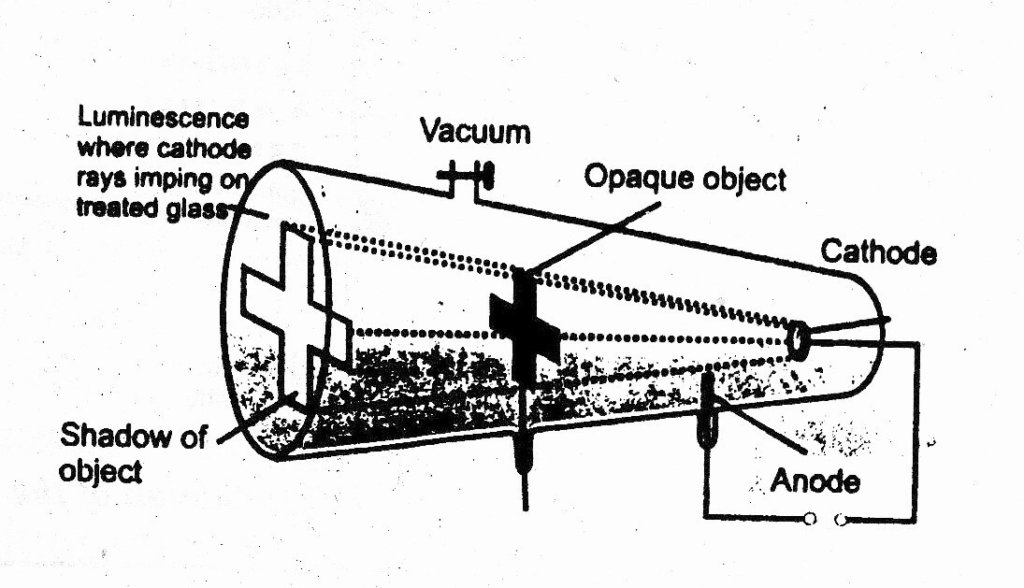
2. Cathode rays produce a shadow when falls upon an opaque. This proves that they travel in a straight line perpendicular to the surface of cathode.
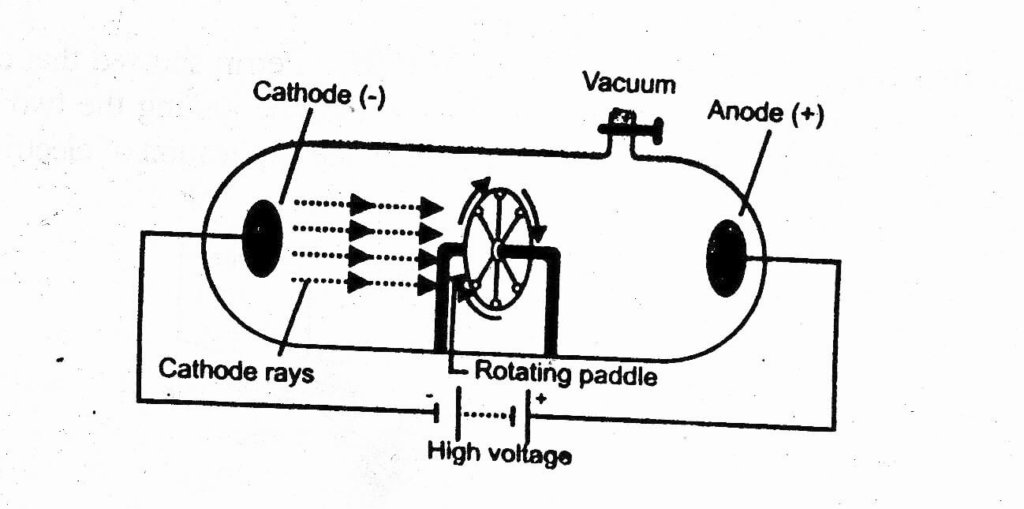
3. These rays can drive a small pedal wheel placed in their path. This shows that these rays possess momentum. So verified that cathode rays are material particles possessing mass & velocity.
4. Cathode rays can produce X-rays when they strike an anode, particularly with large atomic mass.
5. Cathode rays can produce heat when they fall on matter e.g. when cathode rays from a concave cathode are focused on platinum foil, it begins to glow.
6. Cathode rays can ionize gases.
7. They can cause a chemical change, because they have a reducing effect.
8. Cathode rays can pass through a thin metal foil like aluminium or gold foil.
J.J. Thomson concluded from his experiments on cathode rays that these rays consist of streams of negatively charged particles. Stoney named these particles as electrons. The value for e/m remained the same no matter which gas was used in the discharged tube. He concluded that these are not atoms but part of atoms and not the part of a particular atom but part of every atom.



