“The solids having metallic bonds in them are called metallic solids.”
Electron Gas Theory:
The first theory of metallic bonding is called electron pool or electron gas theory. This theory was proposed by Drude and extended by Loren (1932). According to this theory, each atom in a metal crystal loses all of its valence electrons. These valence electrons from a pool or a gas.
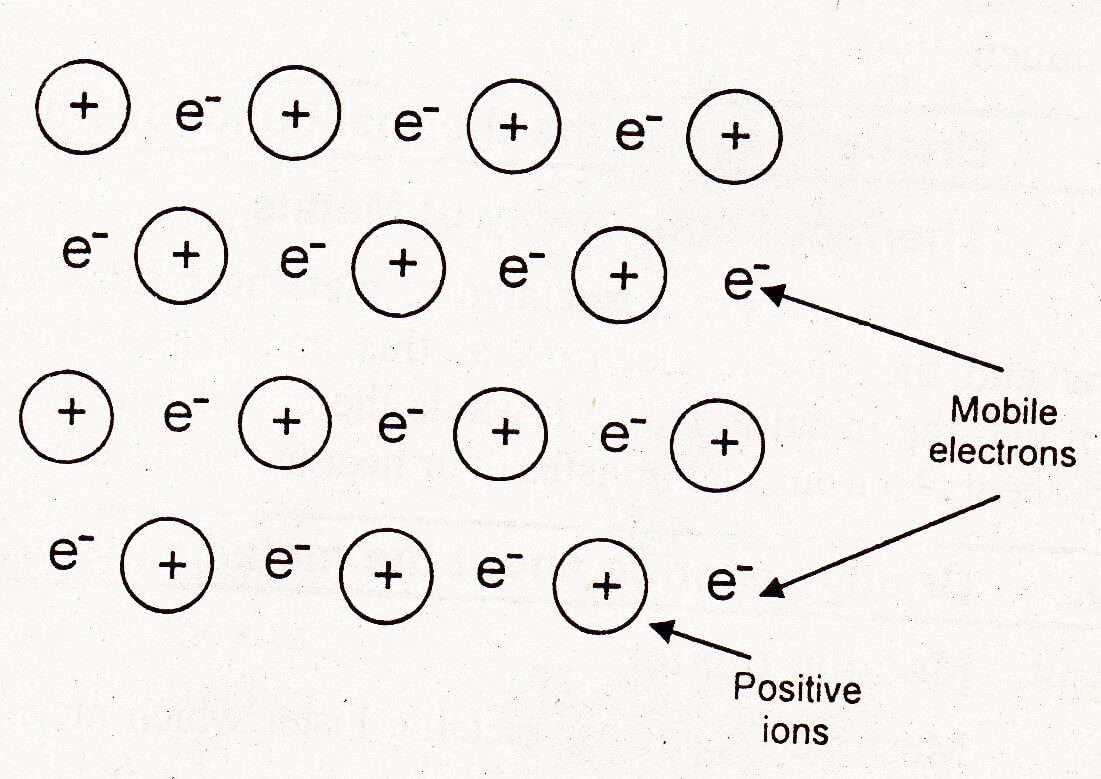
The positively charged metal ions are believed to be held together by electron pool or gas. These positively charged ions occupy definite positions at measurable distances from each other in crystal lattice. Valence electrons are not attached to any individual ion or a pair of ions belongs to the crystal as a whole. These electrons are free to move about from one part of the crystal to the other.
Metallic Bond:
“The force, which binds a metal Cation to a number of electrons within its sphere of influence, is known as metallic bond.”
Metallic Luster
Most metals possess metallic luster which means that they have a shining surface. When light falls on the metallic surface, the incident light collides with the mobile electrons and they are excited.
These excited electrons come back to the original position and give off some energy in the form of light. This light appears to be reflected from the surface of the metal which gives a shining look. Metals are malleable and ductile whenever stress is applied on them.



