The process in which atomic orbitals of different shapes and energy mix and form an equivalent number of new atomic orbitals of the same shape and energy is known as Orbital Hybridization. These new orbitals are known as Hybridized orbitals.
Hybridization’s process got its consideration when some problems were faced in the case of the bonding of some atoms. For example, the electronic configuration of carbon is;
6C = 1S2, 2S2, 2P2
K = 2, L = 4, (2 paired and 2 unpaired)
C has only 2 unpaired electrons in its valence shell, so it should always form only two bonds according to valence bond theory.
However, in all organic compounds, C has formed four covalent bonds. It is because of the transference of one of the 2S electron to the empty 2P. Thus, due to a greater number of unpaired electrons (4 unpaired electrons), C gets more energetic and acquire the excited state (high-energy state).
6C = 1S2, 2S2, 2P2 (Ground state (low energy state) electronic configuration)
6C = 1S2, 2S1, 2P3 (Excited state electronic configuration)
Now C can form 4 covalent bonds. However, as in all the 4 bonds, one S orbital is involved whose shape is different from P orbitals. In addition, its energy is lower (being closer to the nucleus) than P orbitals, thus all 4 bonds C will not be the same (same length and energy).
Therefore, to avoid this problem and to form all bonds of the same energy and length, carbon undergoes the process of Hybridization after exciting and bond formation.
The shape of a hybrid orbital is two-lobed; one lobe is larger while the other one is smaller. The energy of a hybrid orbital is less than the energy of P orbitals and larger than the energy of S orbitals, so we can say that the hybrid orbitals are closer to the nucleus than P orbitals, while away from the nucleus as compared to S orbitals.
Types of Orbital Hybridization
Although there are several types of Orbital Hybridization, the following three are the most simple and main types.
1) SP3 Hybridization
2) SP2 Hybridization
3) SP Hybridization
1) SP3 Hybridization:
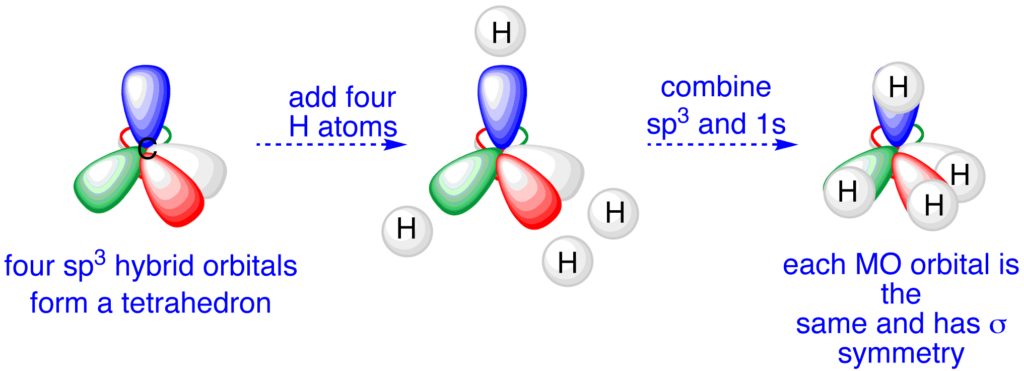
The Hybridization in which one S and three P orbitals intermix and form four hybrid orbitals of the same shape and energy is known as SP3 Hybridization. Each hybrid orbital is known as an SP3 hybrid orbital.
In each SP3 hybrid orbital, the S character is only 25%, while the P character is 75%. Therefore, these 4 SP3 hybrid orbitals will not be very close to the nucleus as they will be near P and away from S orbital.
These four SP3 hybrid orbitals arrange themselves in a tetrahedral geometry. The angle between any two SP3 hybrid orbitals is 109.5o.
Example:
Methane (CH4) molecule:
In the methane (CH4), carbon undergoes SP3 Hybridization; therefore, CH4 has a tetrahedral geometry with a bond angle of 109.5o.
6C = 1S2, 2S2, 2P2 (Round state)
6C = 1S2, 2S2, 2P3 (Excited-state)
2) SP2 Hybridization:
The Hybridization in which one S and two P orbitals of different shapes and energy mix to form 3 hybrid orbitals is known as SP2 Hybridization. Each hybrid orbital is known as SP2 hybrid orbital. In each SP2 hybrid orbital, the S character is 33.33%, while the P character is 66.66%.
Therefore, each SP2 hybrid orbital will be closer to the nucleus than the SP3 hybrid orbitals due to the S character’s increase. Hence, the sigma bond formed from SP2 hybrid orbital will be stronger than the sigma bond formed by SP3 hybrid orbitals.
Thus, the SP2 hybridized carbon will more electronegative than the SP3 hybridized carbon. The three SP2 hybrid orbitals arrange themselves in a “coplanar geometry.” The angle between any two SP2 hybrid orbitals is 120o.
Example:
Ethylene or Ethene Molecule (C2H4):
In the Ethene molecule, each carbon undergoes SP2 Hybridization. Thus, the geometry of the Ethene molecule is coplanar, and the bond angle is 120o. Two of the 2P orbitals (i.e., 2Px & 2Py) undergo Hybridization, and their shape and energy changes.
However, the third orbital, i.e., (2Pz), remains unhybrid. Hence, its shape and energy remain unchanged, and it lies perpendicular to the plane of hybrid orbitals with one lobe above and one lobe below the hybrid orbitals.
Thus when the SP2 hybrid orbital of one C overlaps with the SP2 hybrid orbital of other carbon, a sigma bond is formed between the two carbon atoms.
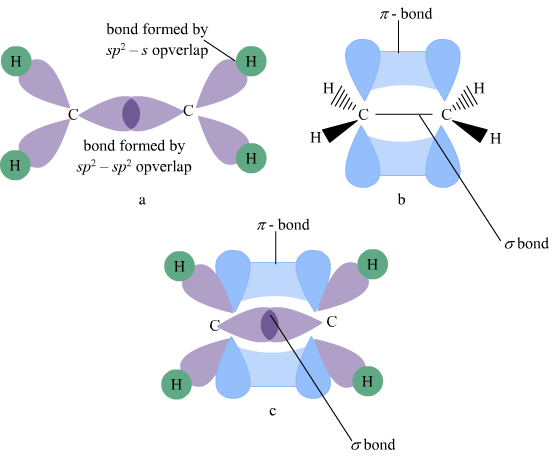
The formation of a sigma bond brings them so close to each other that the unhybrid 2Pz orbital of both the carbon atoms undergoes a sidewise overlap resulting in the formation of a π bond.
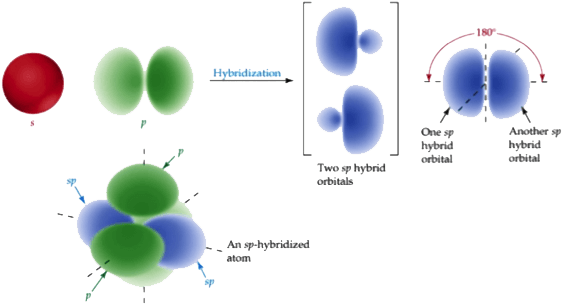
3) SP Hybridization:
The Hybridization in which one S and one P orbital of different shapes and energy mix and form two SP hybrid orbitals of the same shape and energy is known as SP Hybridization.
In each SP hybrid orbital, there is 50% S and 50% P character. Thus, due to the increased S character in SP hybridization, each SP hybrid orbital is closer to the nucleus of the hybridized atom than SP2 & SP3 hybrid orbitals. The SP hybrid orbitals arrange themselves in a linear geometry, and the angle between them is always 180o.
Example:
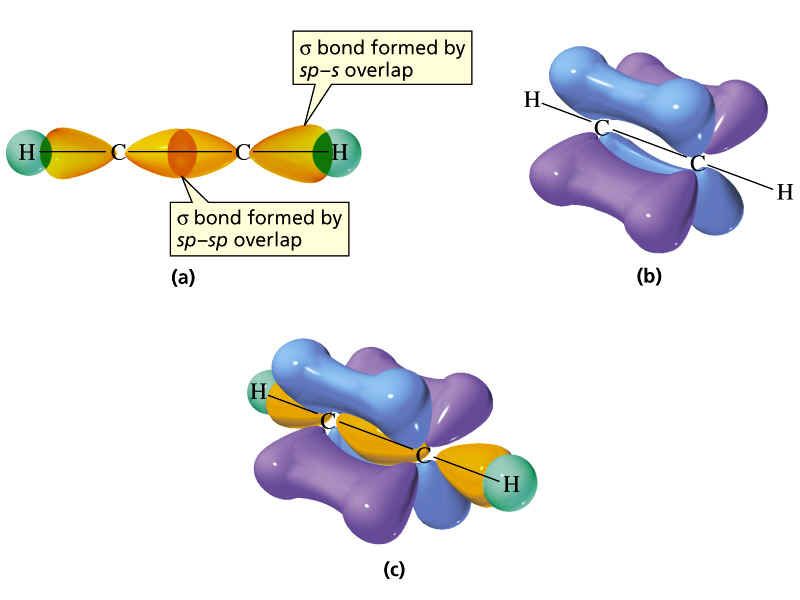
Acetylene or Ethyne (C2H2):
In the acetylene molecule, each carbon undergoes SP hybridization. Thus, the geometry of acetylene is linear, and its bond angle is 180o. As in SP hybridization, one S and only one P orbital are hybridized. Thus, the two P orbitals, i.e., 2Py & 2Pz orbitals of both the carbon atoms remain unhybrid.
The unhybrid orbitals are perpendicular to the plane of hybrid orbitals as well as mutually perpendicular. The one SP hybrid orbital of one carbon atom overlap with the SP hybrid orbital of the other carbon atom forming a sigma covalent bond between them. Similarly, the other SP hybrid orbital of each carbon overlap with the S orbital of hydrogen atoms producing two C – H sigma covalent bonds.
The sigma bond formation between carbon atoms brings them close to each other that the 2Py of one carbon undergoes a sidewise overlap with the 2Py of the other carbon.
The same happens for the 2Pz orbital of both the carbon atoms, thus producing two pi bonds between carbon atoms. Thus in the acetylene molecule, there is a triple covalent bond between the carbon atoms.
Out of these three bonds, one is a sigma covalent bond, while the remaining two are pi (π) covalent bonds. As the sigma bond is formed due to the linear overlap of the orbitals of carbon atoms, it is much stronger. While the pi bonds are of equal strength and formed by the sidewise overlap of the orbitals of carbon atoms; therefore, they are much weaker than the sigma bond.