-
Geometrical Shape Crystalline Solids
Due to definite and orderly arrangement of atoms, ions or molecules in three-dimensional space, all the crystalline solids have a definite, distinctive geometrical shape. For a given crystal, the interfacial angles, at which the surfaces intersect, are always the same no matter in which shape they are grown. The faces and angles remain characteristic even when the material is ground to a fine powder.
-
Melting Points Crystalline Solids
Crystalline solids have sharp melting points and can be identified from their definite melting points.
-
Cleavage Planes Crystalline Solids
The plane surfaces formed by the breaking of a crystal are known as crystal cleavage planes. The angle between the adjacent cleavages of a crystal of a substance are the same, but are different in the crystals of different substances. In other words, these angles are characteristics of crystalline solids.
-
Anisotropy Crystalline Solids
The crystals show variation in physical properties depending upon the direction are called anisotropic properties and the phenomenon is referred to as anisotropy.
Examples:
The physical properties of crystalline solids like refractive index, coefficient of thermal expansion, electrical and thermal conductivities are sometimes anisotropic in nature for some crystals. The variation in these properties with direction is due to fact that the orderly arrangement of the particles in crystalline solids is different in different directions. For example, electrical conductivity of graphite is greater in one direction than in another. Actually electrons in graphite are mobile for electrical conduction parallel to the layers only. Similarly, cleavage itself is an isotropic behavior.
-
Symmetry Crystalline Solids
The repetition of faces, angles or edges when a crystal is rotated by 360°along its axis is called symmetry.
This is an important property of the crystal and there are various types of symmetry elements found in crystals like center of symmetry, plane of symmetry and axis of symmetry.
-
Habit of a Crystal
The shape of a crystal in which it usually grows is called habit of a crystal.
Crystals are usually obtained by cooling the saturated solution or by slow cooling of the liquid substance. These are formed by growing in various directions. If the conditions for growing a crystal are maintained, then the shape of the crystal always remains the same. If the conditions are changed the shape of the crystal may change. For example, a cubic crystal of NaCl becomes needle like when 10% urea is present in its solution as an impurity.
-
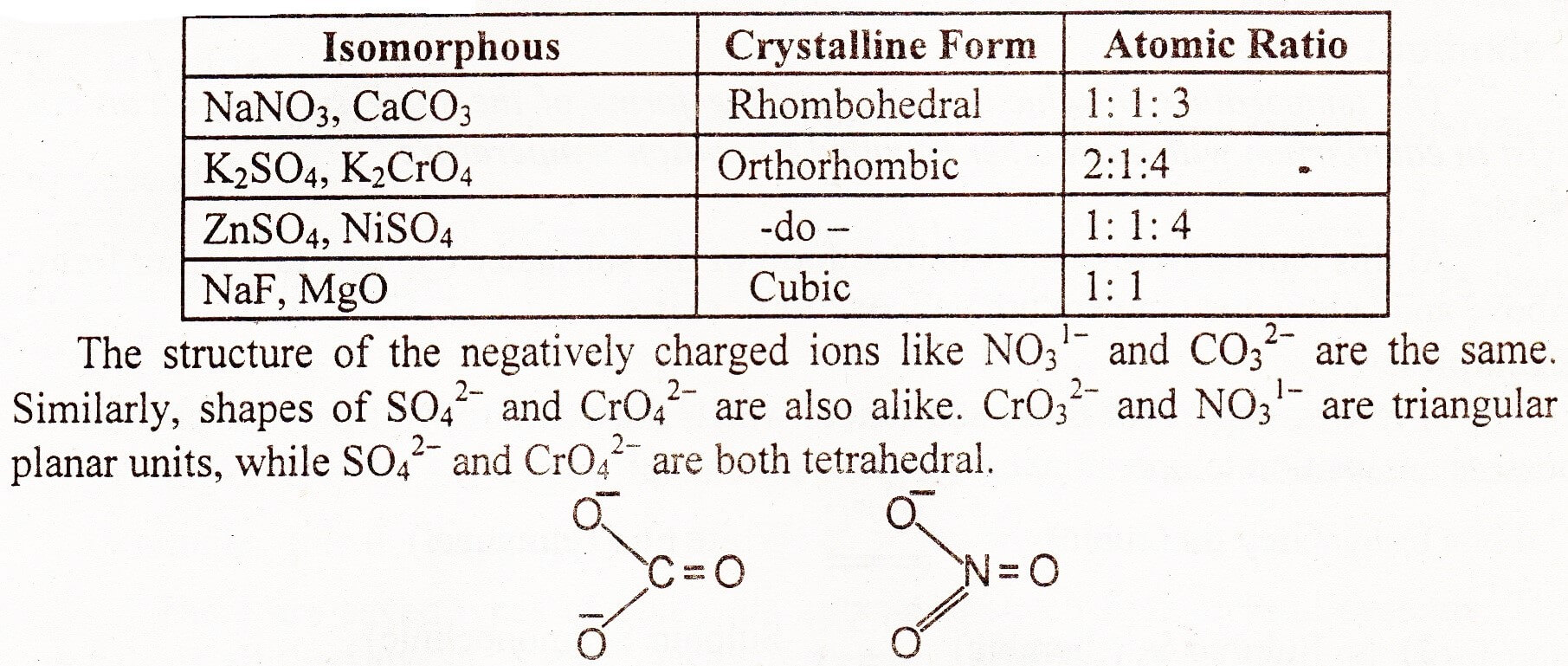
Isomorphism Crystalline Solids
“Existence of two different compounds in same crystalline form is called Isomorphism. These different substances are called isomorphous of each other.”
Explanation:
A crystalline form is independent of the chemical nature of the atoms and depends only on the number of atoms and their way of combinations.
Mostly the ratio of atoms in various compounds is such that isomorphism is possible. Their physical and chemical properties are quite different from each other. Anyway, isomorphic substances crystallize together in all proportions in homogeneous mixtures. Following examples tell us the nature of the compound, their crystalline forms and the ratio of the atoms:
-
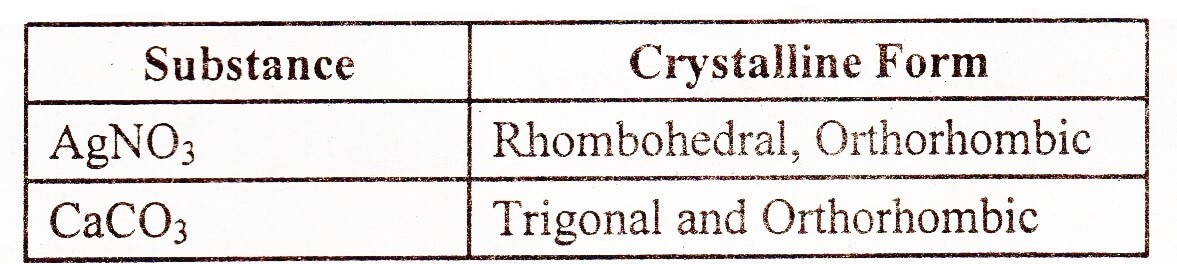
Polymorphism Definition:
The substances which exist in more than one crystalline forms to each other are called polymorphs, and the phenomenon is called polymorphism.
Explanation:
Polymorphs have same chemical properties but they differ in the physical properties. The difference in the physical properties is due to different structural arrangement of particles in space. The following compounds are important polymorphs:
-
Allotropy
When an element exist in more than one crystalline form, this is called allotropy and crystalline forms are called allotropes or allotropic forms. Sulphur, phosphorus, carbon and tin are some important examples of elements which show allotropy.

-
Transition Temperature Definition:
The temperature at which two crystalline forms of the same substance can coexist in equilibrium with each other is called transition temperature.
Note:
At this temperature one crystalline form of the substance changes to another form. Above and below this temperature only one form exists. Examples:
A few examples for those substances which show allotropy or polymorphism and possess a transition temperature are given below:
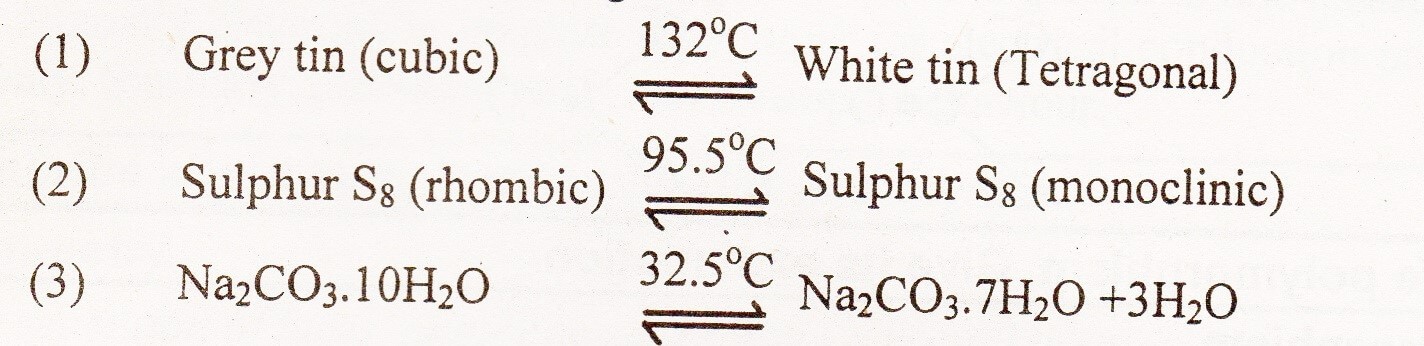
Transition temperature of allotropic forms of an element is always less than its melting point.



