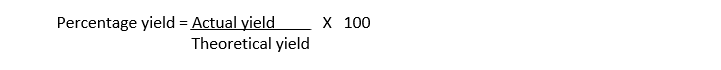The amount of product is termed as yield.
Theoretical Yield:
The amount of the product that is expected to be formed according to the calculations based on balanced chemical equation is called theoretical yield.
- It is the stoichiometric amount of the product.
- It does not require any experimental work.
- It is always greater than actual yield.
Actual Yield:
The weighed mass or measured volume of the product formed as a result of a reaction is called actual yield.
- It is the non-stoichiometric amount of product.
- It requires experimental work.
- It is often less than the theoretical yield.
This is because,
- Sometimes whole of the reactant do not convert into product (as in reversible reaction).
- Sometimes, some side reactions occur and they use up some of the reactants
or products.
- Sometimes some of the product is lost or spilled in handling.
Percentage Yield:
The ratio of actual yield to theoretical yield expressed as percentage is called percentage yield.
It measures the efficiency of the reaction.
Percentage Yield formula: