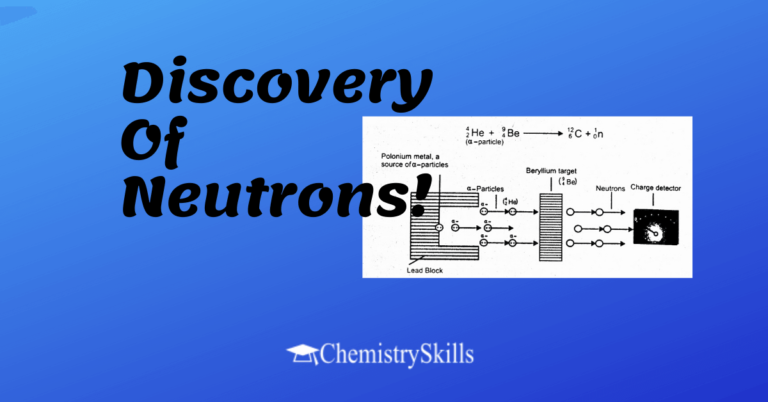
Covalent Solids Definition Chemistry | Properties
“Covalent solids are also called atomic solids because they are composed of neutral atoms of the same or of different elements. These atoms are held together by covalent bonds:”
- When the covalent bonds give joint molecules like diamonds, silicon carbide or aluminium nitride.
- When atoms join to form the covalent bonds and separate layers are produced like that of graphite, cadmium iodide and boron nitride.
Properties of Covalent Crystals
(1) Structure:
These crystals are extended in three dimensions and the valencies of atoms are directed in definite directions, so the packing of atoms in these crystals is looser than that of ionic and metallic crystals. Thus covalent crystals have open structure.
(2) Hardness:
Covalent crystals are very hard and considerable amount of energy is required to break the bond between them. They have highly melting points and their volatility is very low.
(3) Conductivity:
Due to the absence of free electrons and ions they are bad conductor of electricity. However, graphite has a layered structure and the electrons are available in between the layers. These electrons become mobile and conductivity becomes possible.
(4) Solubility:
Mostly covalent crystalline solids are insoluble in polar solvents like water but they are readily soluble in non-polar solvents like benzene and carbon tetrachloride. The covalent crystals having the joint molecules like diamond and silicon carbide are insoluble in all the solvents. Because of their big size, they do not interact with the solvent molecules.
(5) Chemical Reactivity:
The chemical reactions of such crystalline solids are very slow.