The protons and electrons were discovered in 1886 and their properties were completely determined till 1895. Rutherford predicted in 1920 that some kind of neutral particles having mass equal to proton must be present in an atom. Chadwick discovered neutron in 1932 and was awarded Nobel Prize in Physics in 1935.
EXPERIMENT
A stream of a-particles produced from a polonium source was directed at beryllium (Be) target. It was noticed that some penetrating radiations were produced. These radiations were called neutrons because the charge detector showed them to be neutral. The nuclear reaction is as follow:
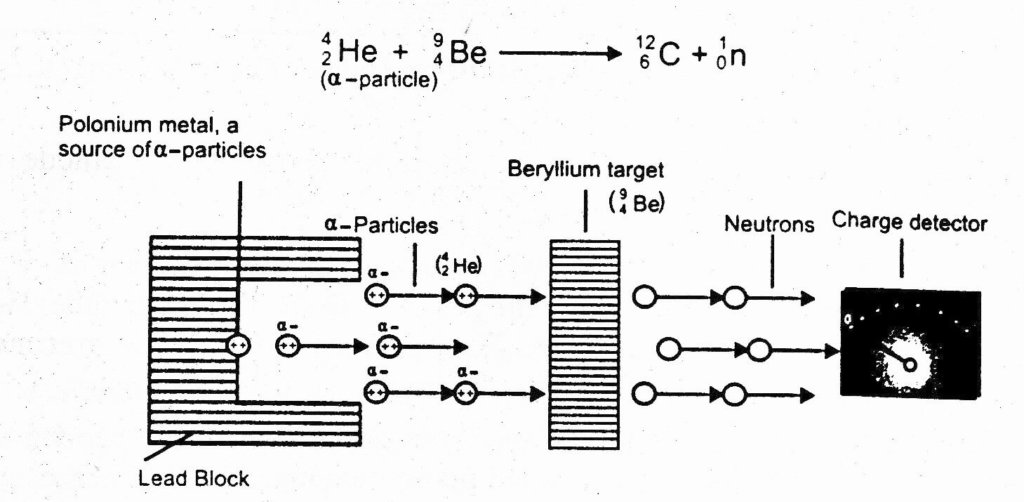
Actually a-particles and the nuclei of Be are rearranged and extra neutron is emitted.
Neutron has a mass of 1.0087 amu while the mass of proton is 1 amu.
Properties of Neutron
- Free neutron decays into a proton with the emission of an electron and a neutrino.
on1 —————-> +1P1 + 1e0 + ono
- Neutrons cannot ionize gases.
- Neutrons are highly penetrating particles.
- They can expel high speed protons from paraffin, water, paper and cellulose.
- When neutrons travel and have the energy 1.2 MeV, then they are called fast neutrons but with energy below 1 eV are called slow neutrons. Slow neutrons are usually more effective than fast ones for the fission purposes.
- When neutrons are used as projectile, they can carry out the nuclear reactions. A fast neutron ejects an -particle from the nucleus of nitrogen atom and boron is produced.
14N7 + 0n1 —————–> 5B11 + 2He4
When slow moving neutrons hit the Cu metal then radiations are emitted. The radioactive Cu is converted into Zn.
29Cu65+ 0n1 ———————–> 29Cu66 + hv
29Cu66 ——————->30Zn66 + -1e0
Because of their intense biological effects they are being used in the treatment of cancer.



