Discovery & Properties of Positive Rays(Canal Rays-Protons)
As the atom was considered neutral, therefore, soon after the discovery of negatively charged particles i.e., electrons, scientists tried to discover a positively charged constituent of atom.
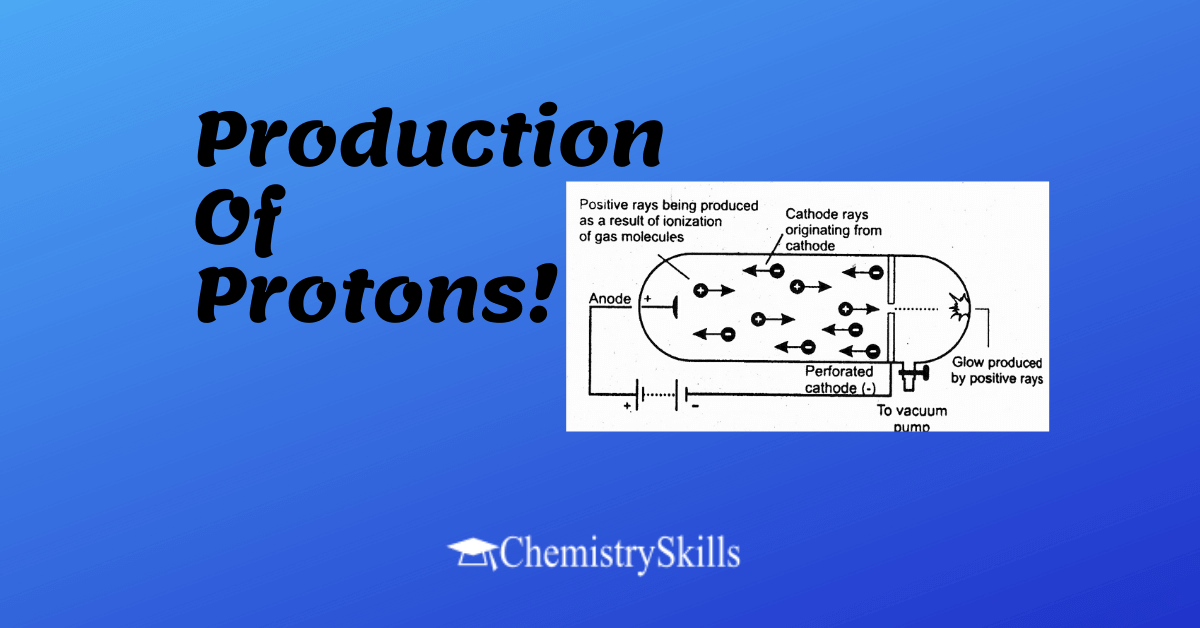
In 1886, Eugene Goldstein used a discharge tube with perforated cathode. He observed a glow in the region behind the cathode. This glow was consequence of rays, which were striking the glass wall after passing through the holes of cathode. These rays were named as canal rays, because they pass through the canal or opening into the cathode. These rays are known as positive rays.
Production of the Positive Rays:
Canal rays or positive rays are the cations, which move towards the cathode and are obtained when cathode rays knock out electrons from the atoms of the gas taken in the discharge tube.
M +e– ® M+ + 2e–
Properties of canal rays or positive rays
i) They are deflected by an electric or magnetic field, showing these are positively charged.
ii) These rays travel in straight line in a direction opposite to the cathode rays.
iii) They produce flashes on ZnS plate.
iv) The e/m value for the positive rays is always smaller than that of electrons and depends upon the nature of the gas used in the discharge tube. Heavier the gas smaller is the e/m value. For hydrogen e/m value is maximum as compared to any other gas. Because hydrogen has the lowest mass.
v) The positive particle obtained from hydrogen is the lightest among all the other positive particles and is called proton having mass 1836 times greater than that of electron.