Charles’ Law
Introduction:
In 1787, Charles found a relationship between volume and temperature of gases. He announced this relationship in the form of a law known as Charles’ law:
Definition:
“Volume of a given mass of a gas is directly proportional to the absolute temperature at constant pressure.”
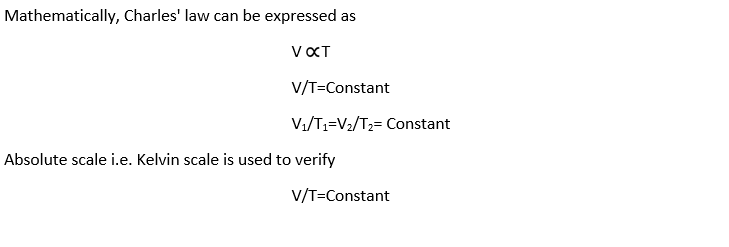
Mathematical Charles Law Formula:
Another Statement for Charles’ Law:
“At constant pressure the volume of a given mass of an ideal gas increases or decreases by 1/273 of its original volume at 0°C for every 1°C rise or fall in temperature respectively”.
Mathematically,
Vf=V0[1+to/273]
Where V0, is the volume of the gas at 0°C and Vf is the volume of the gas at t°C. It is clear from the above expression that at -273°C, the volume of given mass of a gas would be zero.
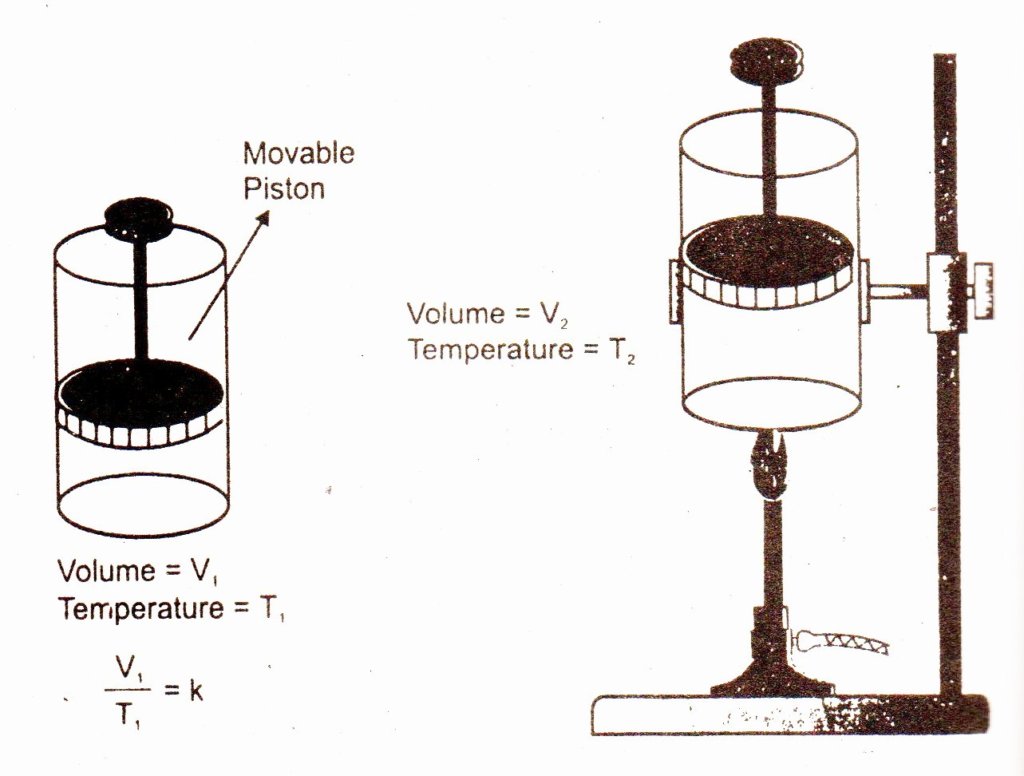
Experimental Verification of Charles’ Law:
The Charles’ Law can be verified by a cylinder having moveable piston. The given mass of a gas is enclosed in the cylinder. Temperature of the gas is increased by heating which causes to increase the volume. By changing the temperature at constant pressure, different volumes are obtained. V/T is always found constant at the same pressure.